Blog

Written by Dean Ihemesie
Introduction
Medications can be powerful tools for managing disease symptoms and progression and for improving our quality of life. However, it's important to remember that even the most rigorously tested drugs come with the potential for adverse drug reactions (ADRs). ADRs are any harmful or unpleasant side effects that may occur when a medication is used as intended, misused, or used off-label. These reactions can range in severity from mild annoyances like an upset stomach, headache, or drowsiness to serious complications such as organ damage or allergic reactions.
There are a few reasons why ADRs occur. Sometimes they are predictable, related to the known effects of the drug, and can often worsen with higher doses. Other times, ADRs are unpredictable, representing an unusual sensitivity or susceptibility to the medication. It's absolutely crucial to be aware of the side effects of any medication you take, and with the support of precision medicine, which can tailor treatment and prevention strategies to an individual, these unpredictable ADRs can be mitigated. In this blog, we will explore ADRs and the burden they present on both the patient and the healthcare system and how that burden can be reduced. Please note that in this blog post, we'll be using the terms "adverse drug reaction" (ADR) and "adverse drug event" (ADE) interchangeably. While there are slight technical differences between the two, we'll use them both to refer to any unintended or harmful effects of medications.
Types of ADRs
ADRs are traditionally classified into two types, Type A and B. Type A reactions are dose-dependent and the side effects are predictable based on drug pharmacology. Type B reactions are idiosyncratic, meaning their occurrence is not explained by the drug's pharmacological profile. Though traditional, these two types are not enough to classify all ADRs. (Coleman et. al. 2016). An alternative to Type A and B is “DoTS”, which classifies ADRs based on Dose of the drug, the Time course of the reaction and relevant Susceptibility factors (such as genetic, pathological, and other biological differences) (Aronson et. al. 2016).
Classification of ADRs is important, because it assists us in identifying patterns, causes, and other potential risks related to medications. By categorizing ADRs, we can discern potential links to a drug's pharmacodynamic properties, target interactions, or patient-specific factors like metabolism and clearance. This knowledge is critical for developing risk prediction models, optimizing dosing regimens, and guiding the design of next-generation therapeutics with improved safety profiles.
Why can unpredictable ADRs occur?
People don't all react to medications in the same way. There are several reasons why some individuals are more likely to experience ADRs than others. Age is one important factor. Young children and older adults tend to be more sensitive to medications. Their bodies are either developing or aging, and they may process drugs differently, making them more prone to side effects. Medications can also cause problems when they mix with each other or even food. (Ferner et. al. 2019). For example, combining certain antidepressants and some migraine medications can lead to a potentially life-threatening condition known as serotonin syndrome, which causes symptoms like agitation, confusion, muscle tremors, fever, and even seizures (Wooltorton 2006). Underlying health conditions, such as liver or kidney disease, can also play a role. These organs are often responsible for breaking down and getting rid of drugs from the body. If these organs aren't working properly, drugs may stay in the system longer and reach higher levels in the body, potentially leading to ADRs.
Polypharmacy individuals are one population that are at an even greater increased risk of ADRs. These individuals tend to be older adults or at-risk, potentially making them more sensitive to certain medications. By definition, these individuals are on five or more medications, leading to the increased possibility of drug-drug interactions. Older adults and at-risk populations are more likely to have underlying health conditions, which can ultimately influence drug metabolism (Malki et. al. 2020).
Genes can also significantly influence how medications are processed. Some people have genetic variations that make them break down certain medications very slowly. These individuals may experience stronger or longer-lasting side effects because their bodies take longer to get rid of the drugs and are known as “poor metabolizers” (PM). On the other hand, some people are "ultra-rapid metabolizers” (UM), meaning their bodies process drugs so efficiently that the medications may not work as well as intended (Kane 2021).
The Economic Burden of ADRs
Adverse drug reactions are a significant contributor to hospital admissions worldwide, with the rates varying considerably depending on the region. In North America, ADRs are estimated to be responsible for 4.2% to 30% of hospital admissions in the USA and Canada. Australia sees a range of 5.7% to 18.8% of hospital admissions attributed to ADRs and in Europe, the range falls between 2.5% and 10.6% (Howard et. al. 2007).
The FDA estimates that there have been approximately 2.1 million ADRs reported for 2023, just over half of those ADRs were classified as serious, and roughly 160,000 resulted in death (FDA Adverse Events Reporting System (FAERS) Public Dashboard, March 2024).
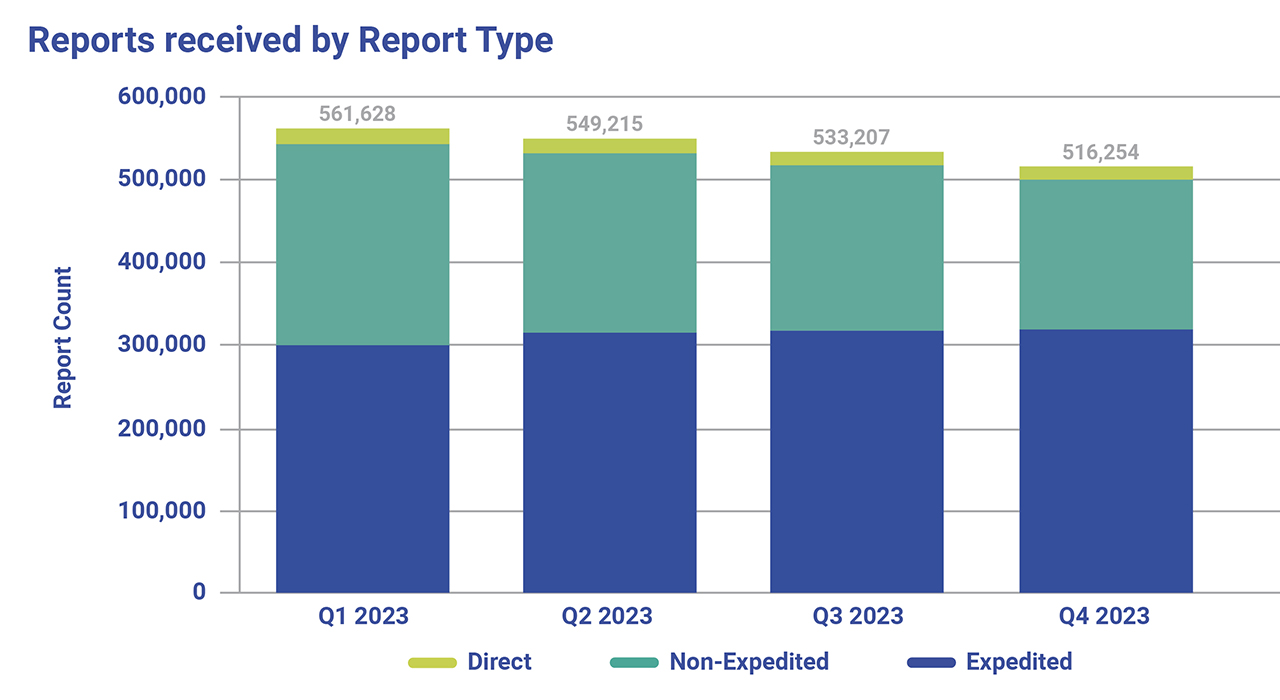
Fig. 1. Summary of Adverse Drug Events reported in 2023 using the FAERS Public Dashboard. Direct Reports are voluntarily submitted directly to FDA through the MedWatch program by consumers and healthcare professionals. Expedited reports contain at least one ADR that is not currently described in the product labeling. Non-Expedited reports are reports that do not meet the criteria for expedited including cases that are reported as serious and expected, non-serious and unexpected, and non-serious and expected.
The total economic burden of ADRs often goes unrealized, obscured within the complexities of a healthcare spending system that can sometimes seem unregulated. While hospitalizations due to severe ADRs get the attention they deserve, the costs can quietly accumulate in countless other ways, such as unnecessary diagnostic tests and the ADRs being perceived as signs of a new illness. Patients may pay for additional medications to manage side effects, or miss workdays seeking relief, impacting their potential income. Family members may take on the financial and logistical strain of providing support. The true cost can even extend beyond dollars, as ADRs can delay treatment of existing diseases and ultimately diminish quality of life.
The financial impact of drug-drug interactions alone on healthcare costs is substantial, with estimates ranging from $30 - $180 billion (Peabody et. al. 2018). The cost of treating adverse drug reactions (ADRs) in hospitals varies significantly depending on the ward. While a patient in a non-intensive care unit (NICU) incurs an average cost of $US 13,994 due to ADRs, this figure jumps considerably to $US 19,685 in intensive care units (ICU) (Sultana et al. 2013). This highlights the potential for increased severity and complexity of ADRs in patients, who may be already critically ill and taking multiple medications, making them more susceptible to adverse reactions. The substantial variation in financial costs emphasizes the complexity of the issue and the need for continued research to develop more effective strategies for preventing and managing ADEs.
Prevention of ADRs and Precision Medicine
Precision medicine is a rapidly evolving approach to healthcare that can tailor treatment and prevention strategies to an individual's specific genetic makeup, environment, and lifestyle. It does this by considering various factors, such as but not limited to, sex, age, underlying issues, and even ethnicity. For example, the National Institute for Health and Care Excellence recommends prioritizing angiotensin-II receptor blockers (ARBs) over angiotensin-converting enzyme (ACE) inhibitors for patients with African or Caribbean descent when treating high blood pressure. This is due to a potentially higher risk of swelling associated with ACE inhibitors in this population (Coleman et. al. 2016).
As we have discussed earlier in this blog, your genes also play a vital role in ADRs. Pharmacogenomics or PGx is a subset of precision medicine that focuses on genomic variation within individuals, and how that variation can affect their response to drug therapy. For example, the DPYD gene encodes dihydropyrimidine dehydrogenase (DPD), an enzyme that catalyzes the rate-limiting step in fluorouracil (5FU) metabolism; with 5FU being a commonly used chemotherapy drug for various cancers. Specific variations in DPYD can result in an enzyme with reduced or non-existent activity, leading to 5FU toxicity, which can often prove fatal (Dean et. al. 2016).
Lastly, communication is essential, patients should always provide a complete medication history (including over-the-counter drugs and supplements) to all their healthcare providers. This transparency allows doctors and pharmacists to catch potential drug-drug interactions or other sensitivities that can often be missed or overlooked. Ultimately, prevention of ADEs requires a team effort between patients, healthcare providers, and technology.
Final Thoughts
Medications are a necessary tool to improve our quality of life, with benefits encompassing a wide range of health conditions. From managing chronic conditions like high blood pressure and diabetes to providing relief from acute pain and even helping to stabilize moods, medications play a crucial role in promoting our overall well-being. It’s important to realize that the risk of adverse drug reactions (ADRs) extends far beyond individual patients. ADRs trigger a cascade of downstream costs. Patients may require extended hospital stays, often for days or even weeks, to manage the ADR itself. This can necessitate additional treatments and potentially even lead to long-term disabilities. This translates to a significant economic burden on the healthcare system, with costs ballooning due to emergency room visits, hospitalizations, and lost productivity.
Investing in preventative measures, such as enhanced patient-provider communication, cutting-edge technologies, and precision medicine advancements, is key to improving overall health outcomes. By taking a more proactive approach rather than a reactive one, we can ensure early detection of potential issues and create personalized treatment plans. Finally, continued advancement in precision medicine will enable us to better treat individual patients based on their unique situation, leading to more effective interventions and improved quality of life.
References
- Coleman JJ, Pontefract SK. Adverse drug reactions. Clin Med (Lond). 2016 Oct;16(5):481-485. doi: 10.7861/clinmedicine.16-5-481. PMID: 27697815; PMCID: PMC6297296.
- Aronson JK, Ferner RE. Joining the DoTS: new approach to classifying adverse drug reactions. BMJ. 2003 Nov 22;327(7425):1222-5. doi: 10.1136/bmj.327.7425.1222. PMID: 14630763; PMCID: PMC274067.
- Ferner R, Aronson J. Susceptibility to adverse drug reactions. Br J Clin Pharmacol. 2019 Oct;85(10):2205-2212. doi: 10.1111/bcp.14015. Epub 2019 Jul 17. PMID: 31169324; PMCID: PMC6783620.
- Wooltorton E. Triptan migraine treatments and antidepressants: risk of serotonin syndrome. CMAJ. 2006 Oct 10;175(8):874. doi: 10.1503/cmaj.061217. Epub 2006 Sep 20. PMID: 16988028; PMCID: PMC1586097.
- Malki MA, Pearson ER. Drug-drug-gene interactions and adverse drug reactions. Pharmacogenomics J. 2020 Jun;20(3):355-366. doi: 10.1038/s41397-019-0122-0. Epub 2019 Dec 3. PMID: 31792369; PMCID: PMC7253354.
- Kane M. CYP2D6 Overview: Allele and Phenotype Frequencies. 2021 Oct 15. In: Pratt VM, Scott SA, Pirmohamed M, et al., editors. Medical Genetics Summaries [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2012-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK574601/
- FDA Adverse Events Reporting System (FAERS) Public Dashboard. 2023. Retrieved March 20th 2023, 02:00 PM ET https://fis.fda.gov/sense/app/95239e26-e0be-42d9-a960-9a5f7f1c25ee/sheet/7a47a261-d58b-4203-a8aa-6d3021737452/state/analysis
- Peabody J, Acelajado MC, Robert T, Hild C, Schrecker J, Paculdo D, Tran M, Jeter E. Drug-Drug Interaction Assessment and Identification in the Primary Care Setting. J Clin Med Res. 2018 Nov;10(11):806-814. doi: 10.14740/jocmr3557w. Epub 2018 Oct 9. PMID: 30344815; PMCID: PMC6188027.
- Sultana J, Cutroneo P, Trifirò G. Clinical and economic burden of adverse drug reactions. J Pharmacol Pharmacother. 2013 Dec;4(Suppl 1):S73-7. doi: 10.4103/0976-500X.120957. PMID: 24347988; PMCID: PMC3853675.
- Howard RL, Avery AJ, Slavenburg S, Royal S, Pipe G, Lucassen P, Pirmohamed M. Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol. 2007 Feb;63(2):136-47. doi: 10.1111/j.1365-2125.2006.02698.x. Epub 2006 Jun 26. PMID: 16803468; PMCID: PMC2000562.
- Dean L, Kane M. Fluorouracil Therapy and DPYD Genotype. 2016 Nov 3 [updated 2021 Jan 11]. In: Pratt VM, Scott SA, Pirmohamed M, Esquivel B, Kattman BL, Malheiro AJ, editors. Medical Genetics Summaries [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2012–. PMID: 28520376.

