Serology Testing
Serology Overview

Incite Health’s serology immunoglobulin response platform may be used to support a range of specialized clinical seroconversion applications for vaccine development or infectious disease screening.
The test works by using a small sample of blood from the patient, which is analyzed for the presence of different types of antibodies that target specific antigens. Two main types of antibodies that can be detected are IgM and IgG. IgM antibodies are typically the first type of antibodies to appear in response to an infection or vaccination, while IgG antibodies are produced later and are believed to provide long-lasting immunity.
SARS-CoV-2 Serology Test

The first iteration of this is the Incite Health multiplexed serology screen for immunoglobulins targeting antigens of the SARS-CoV-2 virus that causes COVID-19. This test enables individuals to discern if they have been exposed to the virus and/or understand how they have responded to Covid-19 vaccination over time.
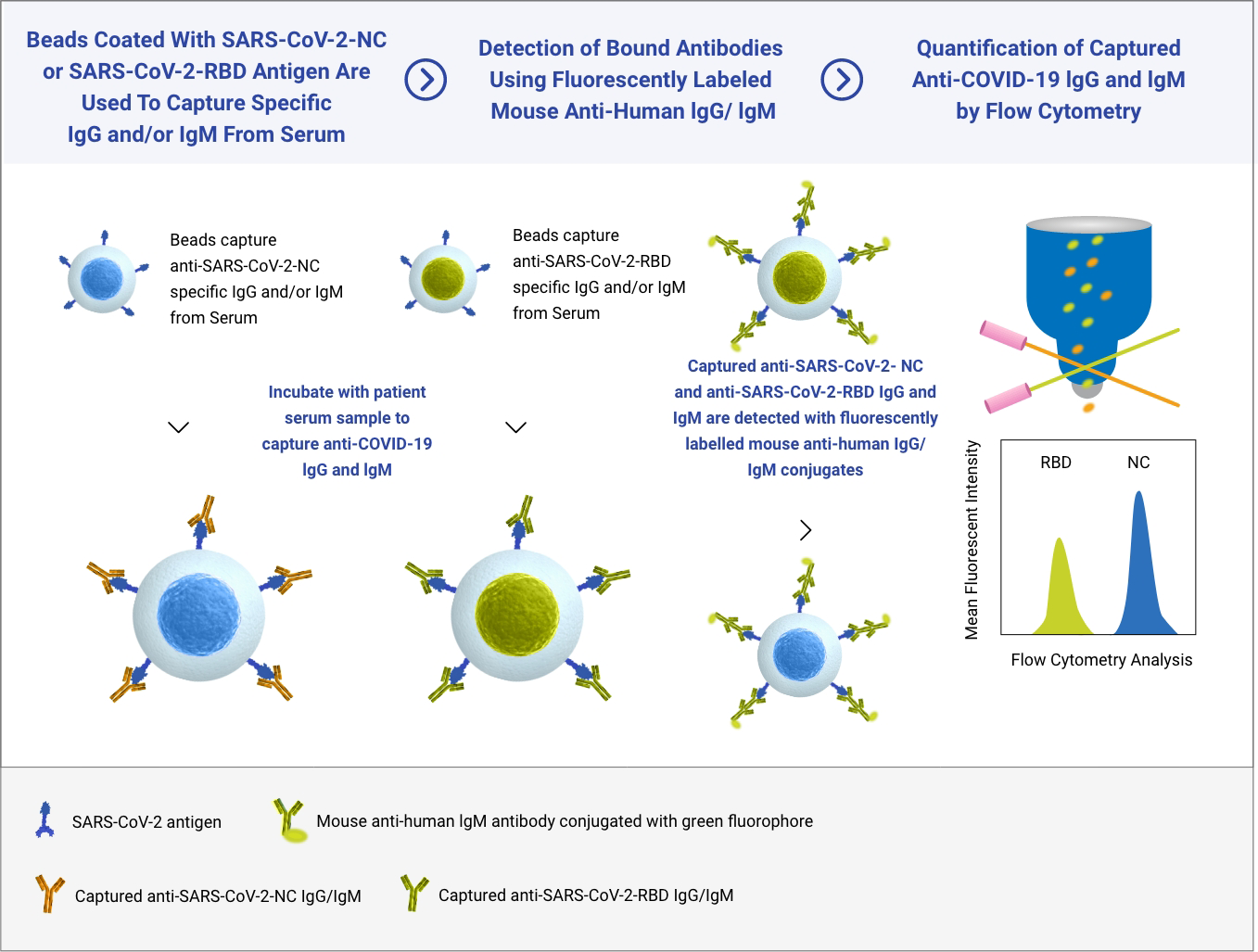
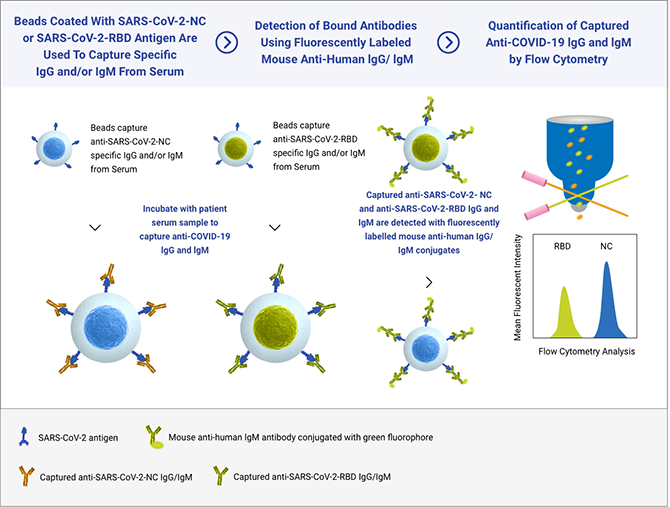
Incite Health’s SARS-Cov-2 serology test was established on a Flow Cytometry platform supporting multiplexed antibody detection targeting two separate SARS-CoV-2 specific antigens: the highly immunogenic Nuclear Capsid (N) protein and the Receptor-Binding Domain (RBD) portion of the Spike (S) protein, which is associated with Neutralizing Antibodies, and the target of the vaccines approved by the FDA.
Our technology simultaneously detects antibodies that target two COVID-19 viral proteins. Our SARS-Cov-2 Immune Response Test provides both a qualitative (positive or negative) result and a semi-quantitative (antibody amount assessment) which means an individual’s immune response to the COVID-19 vaccine or to prior exposure to the SARS-CoV-2 virus.
the highly immunogenic Nucleocapsid (N) protein

the Receptor-Binding Domain (RBD) portion of the Spike (S) protein, which is associated with neutralizing antibodies
Incite Health’s SARS-CoV-2 Serology Test Performance Highlights

Multiplexed approach with selected recombinant antigens minimizes potential cross-reactivity with other viral antigens.
Provides higher positive predictive values across a wide dynamic range of immunoglobulin levels.
Demonstrates high performance >99% clinical sensitivity and specificity.
Supports complete immune-response profile of individuals to SARS-CoV-2 infection, Covid-19 vaccination or the combination of both infection and vaccination.
Applications of the Multiplexed Serology Test Platform

Enables the semi-quantitative measurement of IgM and IgG responses independently targeting multiple antigens in the same sample.
Uniquely capable of detecting immune responses to specific antigen targets over a wide dynamic range of immunoglobulin levels, assessing the immunogenicity of these antigens independently.
Highly sensitive to enable early immune responses, even within immuno-compromised patients to be semi-quantified and tracked overtime.
Enables the optimal adoption of vaccination and booster protocols for different patient cohorts.
Robust high-throughput platform facilitates the monitoring of large cohorts and tracking of titers overtime.