Blog

Written by Julie Bick, Ph.D.
One of the most promising areas of precision medicine is the field of pharmacogenomics (PGx), a discipline that explores the relationship between an individual's genetic makeup and their response to medications. While PGx has traditionally been associated with adult populations, its application in pediatric patients is becoming increasingly recognized as a valuable tool for guiding treatment decisions. The adoption of pediatric pharmacogenomic screening for newborns is now becoming more and more commonplace, with PGx testing now being incorporated as the standard of care within world famous pediatric institutions including Nicklaus Children’s Hospital, Florida (Nicklaus Children’s Hospital is the first Pediatric Hospital in South Florida to Adopt Innovative Ph); Children's Mercy, Kansas (Pharmacogenetic Testing) and Children's Hospital Boston (Genomics for Personalized Safer Medication Choices for Kids). St. Jude's Children Hospital is now incorporating PGx into their clinical trials (PG4KDS: Pharmacogenetics Clinical Trial) to help stratify cohorts. The idea that one test will provide a lifetime of value to an individual is a powerful argument for the widespread adoption of pediatric PGx testing, providing benefits for all stakeholders. In this blog post, we examine clinical practices that have incorporated PGx testing for pediatric patients, exploring its significance, challenges, and the transformative impact it has on tailoring medical interventions for young individuals.
Understanding PGx in the Context of Pediatric Care
PGx is the study of how an individual's genetic makeup influences their response to drugs. The variation in genes responsible for drug absorption, metabolism, transport, excretion, and target interactions can significantly impact the efficacy and safety of medications. By identifying genetic markers that influence drug response, healthcare providers can personalize treatment plans to maximize benefits and minimize adverse effects. In the context of pediatric patients, understanding the genetic factors that influence drug metabolism is crucial. It is well documented that children may metabolize drugs differently than adults due to variations in enzyme activity, organ function, and growth rates. Research has shown that the activity of several genes central to standard PGx testing are developmentally driven; key examples include CYP2D6 that is fully expressed by 2-weeks of age (Stevens et. al. 2008), compared with CYP2D19 that may not be fully expressed until 10 years of age (Pierre-François et. al. 2022). But taking such variations of expression into account, PGx testing can assist clinicians by incorporating into a child's unique genetic profile, to make more informed decisions about drug selection, dosage, and treatment duration. Despite examples of clinical relevance, PGx has not been widely adopted in the field of pediatrics for a range of reasons. These include the much smaller patient population that pediatrics represents compared with adult patients, leading to smaller clinical trials, and therefore challenges in the statistical analysis of the PGx data. In addition, the smaller pediatric patient populations are much more likely to be prescribed off-label of unlicensed drugs because there in many cases are no alternatives.
The Value of PGx Testing in Pediatrics
Actionable PGx testing in adults has been proven to be highly effective in detecting the genetic predisposition to an Adverse Drug Reaction (ADR) for some drugs. The same principles apply for pediatric patients, although this population may be more susceptible to adverse drug reactions due to their developing physiology. There are some areas of medicine that are typically associated with pediatric patients, and PGx has proved to be a valuable tool in their implementation into standard of care. This includes drugs used to manage cystic fibrosis or those used in the field of pediatric haemato-oncology for example, and PGx has provided significant value in the optimization of therapeutic approaches used in these examples (Table 1). This type of testing can be either pre-emptive or reactive based on the timing of the testing relative to the start of the therapeutic intervention. There is significant debate over the value versus cost of pre-emptive testing in general, although many agree it actually may prove to be more valuable for pediatric medicine than any other area.
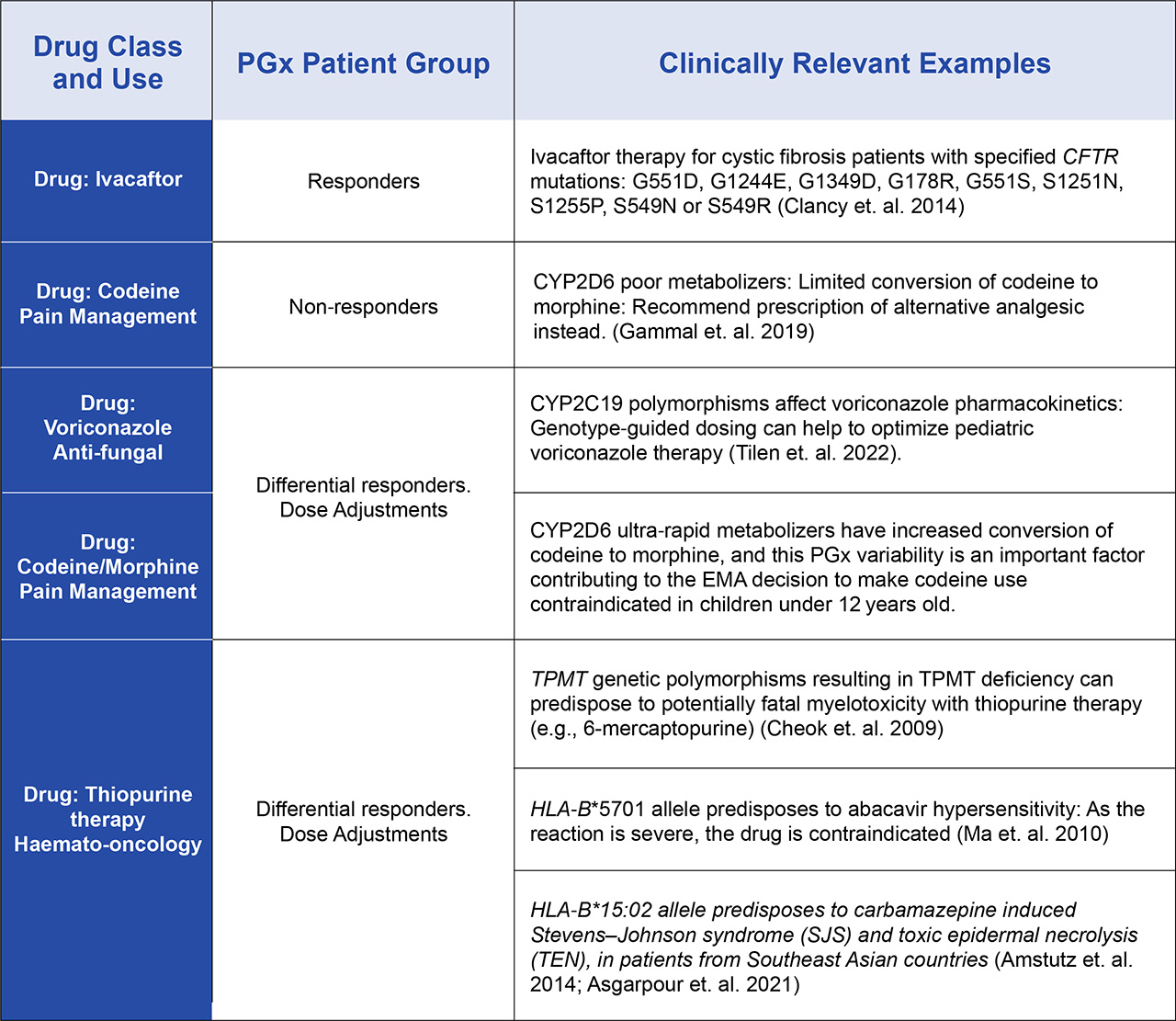
Table 1. Examples of Clinically Actionable PGx stratification of pediatric patient groups that are now being used for therapeutic selection and optimization.
How PGx is Transforming the Management of Treatments for Cystic Fibrosis
Cystic Fibrosis is a genetic disorder that affects a key mucosal protein in the body that functions in many organs to support the respiratory and digestive systems. Cystic Fibrosis used to lead to an early death in childhood, but survival rates have improved with advancements in neonatal screening and more effective therapeutic interventions. Today, worldwide around 100,000 children and adults now live with Cystic Fibrosis, most with life expectancies of over 60 years. PGx testing has identified specific genetic mutations in Cystic Fibrosis patients that respond well to the drug Ivacaftor, leading to improved lung function and quality of life in affected children (see Table 1). However, beyond this, over their lifetimes patients with Cystic Fibrosis are typically prescribed a range of drugs to manage their symptoms and many of these drugs have Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines to optimize dosing. These CPIC guidelines are now driving the recommendation that pediatric PGx testing should be made available as standard of care for all Cystic Fibrosis patients to help both manage the cost of their treatments and more importantly to improve their quality of life (Sakon et. al. 2021; Anderson et. al. 2023).
How PGx Is Being Used to Improve the Safety of Treatment for Acute Lymphoblastic Leukemia (ALL)
Another example of the potential of PGx to improve pediatric medicine is in the case of ALL, a type of cancer of the bone marrow in which too many immature lymphoblasts are generated. With modern treatment, the 5-year survival rates for children 0 to 14 have increased over the last 40 years from nearly 0% to around 92% today. As far as clinical profiling of patients, Minimal Residual Disease (MRD) is the key prognostic factor in pediatric ALL, and PGx testing has proved valuable in understanding how pediatric patients will respond to antileukemic drugs. Most pediatric ALL patients receive thiopurine-based chemotherapy (mercaptopurine and thioguanine) and PGx testing has revealed genetic variants of the TPMT genes that are associated with higher risk of severe thiopurine-induced toxicities, allowing for personalized dose adjustments and the minimizing of treatment-related complications. In addition, antifolate methotrexate (MTX) is also commonly used to treat pediatric ALL; and there are several genes associated with the uptake and metabolism of MTX. PGx testing is now commonly used to assess the genomic basis of MTX resistance and optimize dosing efficacy (Rau et. al. 2006; Hulot, et. al. 2005; Krajinovic et. al. 2004)
The Use of PGx Profiling to Support the Management of Attention-Deficit/Hyperactivity Disorder (ADHD)
No area of medicine has benefited more from PGx testing than psychiatry and the management of mental health disorders. Within the pediatric population, ADHD is the most prevalent neuropsychiatric disorder, with reportedly around 5.4% of children receiving an ADHD diagnosis (Polanczyk et. al. 2014). Stimulant medication is the first line of drug-intervention and currently there are several medications for childhood ADHD across two drug classes: stimulants/amphetamine (methylphenidate, amphetamine) and non-stimulants (atomoxetine, extended-release versions of guanfacine and clonidine) (Ref American Academy of Pediatrics. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 2011; 128: 1007–1022). Since it is largely considered safe and effective, methylphenidate has become the most prescribed medication for ADHD. However, an estimated 35% of children do not respond to the drug (Hodgkins et. al. 2012) and 3% experience serious adverse reactions, with the development of tics, depression, anxiety, psychosis and or mania (Efron et. al. 1997). Recent retrospective studies have profiled Single Nucleotide Polymorphisms (SNPs) within several genes including CES1A1, SLC6A2, SLC6A3, COMT, DRD4 (promoter duplication) and ADRA2A that influence the response to methylphenidate (Myer et. al. 2017). This information is now guiding clinicians in selecting the most effective medication and dosage for individual pediatric patients, and most importantly, helping to avoid medications that may be harmful and/or ineffective.
Beyond ADHD PGx for Pediatric Mental Health Treatments
There is no doubt that children today are facing a myriad of challenges that are contributing to an increased risk of mental health issues. These include the increased use of social media and technology, academic pressure and bullying at school, as well as increased rates of reported trauma and adverse childhood experiences. Children with a family history of mental illness are at an increased risk of developing similar conditions themselves due to genetic pre-dispositions. The Covid-19 pandemic was associated with increased rates of mental health disorders in pediatric populations, however studies have demonstrated that many individuals suffering during this time either did not seek or did not receive treatment to improve their mental health (Chua et. al. 2021) Sadly, it is common that pediatric patients seeking mental health therapy may undergo multiple medication trials before finding an effective treatment, exposing them to potential risks and prolonging their suffering. The hope now is that the widespread adoption of PGx testing for mental health patients, especially pediatric patients, will reduce the frequency of the trial-and-error approach to drug prescription and provide a more targeted approach to medication selection and dosing, both saving time and minimizing patient discomfort. The Future of Pharmacogenomic Testing in Pediatric Care There are certainly some unique logistical hurdles associated with the implementation of PGx testing into pediatric care. These include the limited clear pediatric PGx guidelines that are currently available in key clinical areas. Both CPIC and PharmGKB are working with pediatricians to extend PGx clinical research, and associations such as the Electronic Medical Records and Genomics (eMERGE) Network, with sites specifically focused on pediatrics (McCarty et. al. 2011), are developing processes to support collaborations across institutions and provide mechanisms to incorporate standardized pediatric PGx testing into electronic medical records. But there are still many questions around PGx test coding, and who should pay for this type of testing. While most agree that PGx testing in pediatric patients represents a revolutionary step towards personalized medicine, there are still many technological and ethical considerations that need to be addressed. Successful PGx implementation such as in the treatment of conditions like cystic fibrosis, ALL, and ADHD underscore the tangible benefits that this type of pre-emptive testing can bring to young patients. Only when the healthcare sector finds a way to overcome challenges, and foster stronger collaborations among healthcare professionals, researchers, and policymakers, will PGx become the standard of care in pediatric medicine. As we continue this type of clinical research, the future holds the promise of improved therapeutic outcomes, enhanced patient safety, and a paradigm shift in how we approach the healthcare needs of our youngest and most vulnerable population.
References
- Stevens JC, Marsh SA, Zaya MJ et al. Developmental changes in human liver CYP2D6 expression. Drug Metab. Dispos. 36(8), 1587–1593 (2008).
- Pierre-François MJD, Gagné V, Brukner I, Krajinovic M. Pharmacogenetic Expression of CYP2C19 in a Pediatric Population. J Pers Med. 2022 Aug 26;12(9):1383. doi: 10.3390/jpm12091383. PMID: 36143168; PMCID: PMC9504170
- Chua KP, Volerman A, Conti RM. Prescription Drug Dispensing to US Children During the COVID-19 Pandemic. Pediatrics. 2021 Aug;148(2):e2021049972. doi: 10.1542/peds.2021-049972. Epub 2021 Jul 20. PMID: 34285080; PMCID: PMC8344340.
- Clancy JP, Johnson SG, Yee SW, McDonagh EM, Caudle KE, Klein TE, Cannavo M, Giacomini KM; Clinical Pharmacogenetics Implementation Consortium. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for ivacaftor therapy in the context of CFTR genotype. Clin Pharmacol Ther. 2014 Jun;95(6):592-7. doi: 10.1038/clpt.2014.54. Epub 2014 Mar 5. PMID: 24598717; PMCID: PMC4026598.
- Gammal RS, Caudle KE, Quinn CT, Wang WC, Gaedigk A, Prows CA, Haidar CE, Taylor AK, Klein TE, Sangkuhl K, Hankins JS, Crews KR. The Case for Pharmacogenetics-Guided Prescribing of Codeine in Children. Clin Pharmacol Ther. 2019 Jun;105(6):1300-1302. doi: 10.1002/cpt.1260. Epub 2018 Nov 22. PMID: 30467830; PMCID: PMC8168366.
- Tilen R, Paioni P, Goetschi AN, Goers R, Seibert I, Müller D, Bielicki JA, Berger C, Krämer SD, Meyer Zu Schwabedissen HE. Pharmacogenetic Analysis of Voriconazole Treatment in Children. Pharmaceutics. 2022 Jun 17;14(6):1289. doi: 10.3390/pharmaceutics14061289. PMID: 35745860; PMCID: PMC9227859
- Cheok MH, Pottier N, Kager L, Evans WE. Pharmacogenetics in acute lymphoblastic leukemia. Semin Hematol. 2009;46:39–51. doi: 10.1053/j.seminhematol.2008.09.002
- Ma JD, Lee KC, Kuo GM. HLA-B*5701 testing to predict abacavir hypersensitivity. PLoS Curr. 2010 Dec 7;2:RRN1203. doi: 10.1371/currents.RRN1203. PMID: 21151380; PMCID: PMC3000684.
- Amstutz, U et. al. Recommendations for HLA-B*15:02 and HLA-A*31:01 genetic testing to reduce the risk of carbamazepine-induced hypersensitivity reactions. Epilepsia, 55(4):496–506, 2014. https://doi.org/10.1111/epi.12564
- Asgarpour JMS, Lam LM, Vogel TK, Goez HR, Fiorillo L. Human Leukocyte Antigen Gene Testing and Carbamazepine-Induced Toxic Epidermal Necrolysis: A Study of Pediatric Practice. Journal of Cutaneous Medicine and Surgery. 2021;25(1):25-29. doi:10.1177/1203475420952422
- Sakon, C. et. al. Opportunity for pharmacogenomic testing in patients with cystic fibrosis. Pediatric Pulmonology Vol. 57, Is. 4, April 2022. Pp 903-907. https://doi.org/10.1002/ppul.25809
- Anderson JD, Davis BH, Giang G, Jones A, Lee CR, Parker K, Searcy H, Benner K, Limdi NA, Guimbellot JS. Pharmacogenetic actionability and medication prescribing in people with cystic fibrosis. Clin Transl Sci. 2023 Apr;16(4):662-672. doi: 10.1111/cts.13479. Epub 2023 Feb 9. PMID: 36760155; PMCID: PMC10087076
- Rau T, Erney B, Gores R, Eschenhagen T, Beck J, Langer T. High-dose methotrexate in pediatric acute lymphoblastic leukemia: impact of ABCC2 polymorphisms on plasma concentrations. Clin Pharmacol Ther. 2006;80:468–476.
- Hulot JS, Villard E, Maguy A, Morel V, Mir L, Tostivint I, et al. A mutation in the drug transporter gene ABCC2 associated with impaired methotrexate elimination. Pharmacogenet Genomics. 2005;15:277–285.
- Krajinovic M, Lemieux-Blanchard E, Chiasson S, Primeau M, Costea I, Moghrabi A. Role of polymorphisms in MTHFR and MTHFD1 genes in the outcome of childhood acute lymphoblastic leukemia. Pharmacogenomics J. 2004;4:66–72
- Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol 2014; 43: 434–442.
- Hodgkins, P., Shaw, M., Coghill, D. & Hechtman, L. Amfetamine and methylphenidate medications for attention-deficit/hyperactivity disorder: complementary treatment options. Eur Child Adolesc Psychiatry 21(9), 477 (2012).
- Efron D, Jarman F, Barker M. Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: a double-blind, crossover trial. Pediatrics 1997; 100: 662–666.
- Myer NM, Boland JR, Faraone SV. Pharmacogenetics predictors of methylphenidate efficacy in childhood ADHD. Mol Psychiatry. 2018 Sep;23(9):1929-1936. doi: 10.1038/mp.2017.234. Epub 2017 Dec 12. PMID: 29230023; PMCID: PMC7039663.
- McCarty CA, Chisholm RL, Chute CG, et al. The eMERGE Network: a consortium of biorepositories linked to electronic medical records data for conducting genomic studies. BMC Med Genomics. 2011;4:13.

