Blog

Empowering Mothers Using Pharmacogenomic Testing in Postpartum Depression Management
PharmacogenomicsWritten by Julie Bick, Ph.D.
Introduction
Although giving birth is often described as one of the most beautiful experiences a woman can have, for many mothers, the period following childbirth can be marked by overwhelming emotions, fatigue, and, in some cases, postpartum depression (PPD). Postpartum depression is a serious condition that affects around one in seven women in the US after giving birth, and is characterized by feelings of sadness, anxiety, and exhaustion. While the exact causes of PPD are multifaceted and not fully understood, emerging research suggests that genetics plays a significant role in predisposing some women to this condition. But genetics may also hold the key to optimizing the use of medications to address this debilitating condition, and as new treatment options are approved by the FDA, there finally seems to be progress in this somewhat neglected field of medicine.
In recent years, pharmacogenomic testing has emerged as a promising tool in personalized medicine, offering insights into how an individual's genetic makeup influences both their risk of developing certain medical conditions, and their response to medications to manage symptoms more effectively. In the context of PPD, pharmacogenomic testing holds immense potential to improve the success of treatment approaches, providing tailored interventions that are more effective and better tolerated by mothers. This blog explores the role that pharmacogenomics can play in supporting the effective treatment of PPD, highlighting how genetic testing can help identify women most at risk of PPD, and identify the optimal therapeutic strategies to support their path to recovery.
Understanding Postpartum Depression
PPD affects approximately 10-15% of mothers worldwide (Gavin et. al. 2005), making it one of the most common complications of childbirth. The condition can manifest in various forms, ranging from mild mood disturbances to severe depressive episodes and suicidal thoughts. Symptoms typically appear within the first few weeks after delivery but can persist for months or even years if left untreated.
The etiology of postpartum depression is complex, involving a combination of biological, psychological, and social factors. Hormonal fluctuations and premenstrual syndrome (PMS), genetic predisposition, history of depression or anxiety, lack of spousal and social support, and stressful life events are among the many contributors to PPD (Ghaedrahmati et. al. 2017). In addition, smoking (Whitaker et. al. 2007), sedentary lifestyle, and poor diet, in particular a deficiency in vitamin B6 in the diet all increase the risk of a woman developing PPD. However, one aspect that has garnered increasing attention in recent years is the role of genetics in influencing an individual's susceptibility to depression, including PPD (Couto et. al. 2015). Studies have shown that women with a family history of depression are more likely to experience PPD themselves, suggesting a hereditary component to the condition. Additionally, genetic variations related to neurotransmitter pathways, such as serotonin and dopamine, have been implicated in the pathogenesis of depression, including PPD. Genetic profiling of patients with PPD has been used to identify the gene variants associated with an increased risk of developing PPD. The results from several clinical studies across the globe are summarized in table 1. Such studies have identified several genes involved in the transport and metabolism of a wide spectrum of hormones and metabolites, reflecting the underlying complexity of PPD.
Table 1. Genes and Genetic Polymorphisms Associated with an increased Rate of PPD
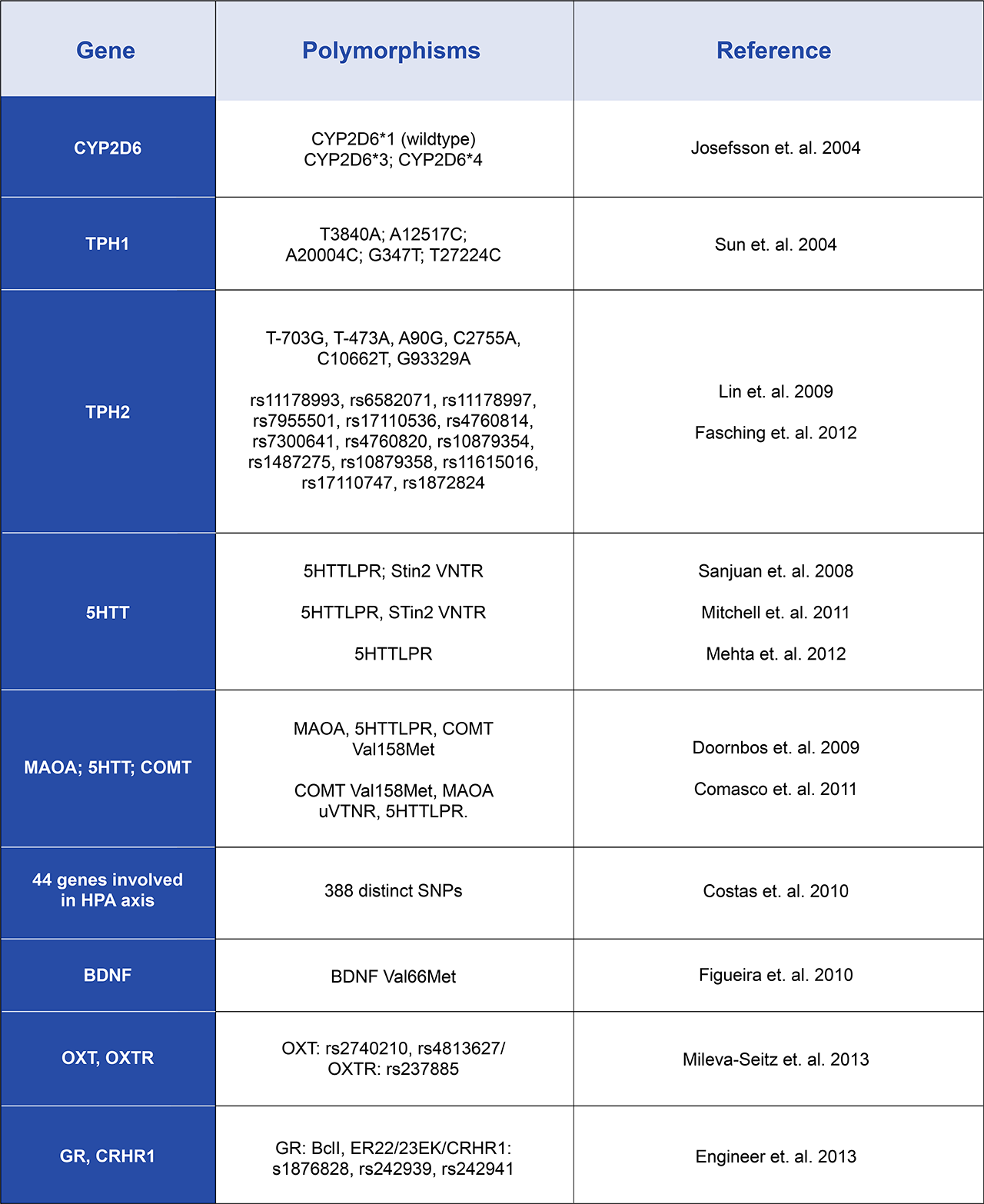
TPH: Tryptophan hydroxylase; 5HTT: Serotonin transporter; MAOA: Monoamine oxidase A; COMT: Catechol-O-methyltransferase; PBMC: Peripheral blood mononuclear cell; BDNF: Brain-derived neurotrophic factor; MTHFR: Methylenetetrahydrofolate reductase; OXT: Oxytocin; OXTR: Oxytocin receptor; GR: Glucocorticoid receptor; CRHR1: Type 1 corticotropin-releasing hormone receptor; ESR1: Estrogen receptor alpha; DRD2: Dopamine receptor D2; HTR2A: Serotonin receptor 2A; DAT: Dopamine transporter.
At its core the pathophysiology of PPD is thought to be caused by changes of both biological and endocrine systems such as the hypothalamic-pituitary-adrenal axis (HPA) resulting in poor or inappropriate stress responses and increased levels of cortisol. The rapid changes in reproductive hormones such as progesterone and estradiol following the birth of the baby may contribute significantly to the onset of PPD. In addition, hormones related to milk production may also be affected, and failure to lactate also frequently coincides with the onset of PPD, and sleep deprivation only contributes to the vulnerability associated with PPD. Sadly, many women do not seek help to address their symptoms, and most women are not pre-emptively offered mental health support after the birth of their babies to help manage the transition to motherhood.
The Treatment of PPD
Typically, the first step to treatment for PPD is talking with a healthcare professional and acknowledging the symptoms. For many women, cognitive-behavioral therapy alone can help break the cycle contributing to the depressive feelings and pave a path to recovery. For other women, medications such as antidepressants that work to rebalance neurotransmitters in the brain help to alleviate symptoms. Some of the most prescribed antidepressant medications for PPD are selective serotonin reuptake inhibitors (SSRI) and these help to maintain adequate serotonin levels in the brain. The downside to these medications is that they can take several weeks to take effect, and not every patient responds the same way to a given antidepressant medication. Finding the right medication can often involve a trial-and-error process that prolongs suffering for mothers experiencing PPD. Pharmacogenomic testing can help streamline this process by identifying antidepressants that are more likely to be effective based on the individual's genetic predisposition. Furthermore, antidepressants are associated with various side effects, which can be particularly concerning for breastfeeding mothers, as these medications can pass into breast milk and affect the baby. Pharmacogenomic testing can identify genetic variations that predispose individuals to risk of adverse reactions to specific drugs, enabling these drugs to be avoided and ensuring the safety of both mother and child (Moretti et .al .2018).
In recent years, the FDA has approved two novel medications specifically to address PPD symptoms: Zulresso (brexanolone) is a neuroactive steroid GABA-A receptor positive allosteric modulator that is administered via an IV infusion over a 60-hour treatment period. Rather than modulating serotonin levels, Zulresso works to restore hormone levels that have shifted following pregnancy. In 2023, the FDA approved the first oral medication specifically for PPD, Zurzuvae (zuranolone). This is taken for 14 days, although the therapeutic effects lasted up to four weeks after treatment concluded. Pharmacogenomic recommendations are not yet in place for either of these medications.
In addition to these new medications, a recent clinical trial has indicated that a single intravenous low-dose (0.25mg/kg) of ketamine, administered post cesarean delivery was highly effective at mitigating PPD (Li and Mei, 2022), and the same positive effect has been demonstrated in similar trials (Han et. al. 2022). This approach is appealing for several reasons- ketamine acts both as an antidepressive and an analgesic, the infusion time is short and the drug is rapidly cleared from the maternal bloodstream, whereas the benefits of treatment are prolonged over several weeks to months. Unlike many other antidepressants including SSRIs, ketamine does not affect breastmilk and there are several published pharmacogenomic markers for improved safety and efficacy (Meshkat et. al. 2022).
Final Thoughts
Postpartum depression can have profound implications for the health and well-being of mothers and their families. For years, this condition has been ignored or minimalized, leading to PPD-related suicides becoming a major cause of maternal death (Oates, 2003). While traditional approaches to treatment have focused on a one-size-fits-all model, pharmacogenomic testing offers a more personalized and targeted approach to managing PPD, and help to both minimize adverse reactions, and improve outcomes for mothers struggling with postpartum depression.
As awareness of the role of genetics in mental health continues to grow, integrating pharmacogenomic testing into routine clinical practice holds great promise for revolutionizing the way postpartum depression is diagnosed and treated. By harnessing the power of personalized medicine, we can now help to empower new mothers to overcome postpartum depression and thrive in their journey through motherhood.
References.
- Ghaedrahmati M, Kazemi A, Kheirabadi G, Ebrahimi A, Bahrami M. Postpartum depression risk factors: A narrative review. J Educ Health Promot. 2017;6:60
- Couto TC, Brancaglion MY, Alvim-Soares A, Moreira L, Garcia FD, Nicolato R, Aguiar RA, Leite HV, Corrêa H. Postpartum depression: A systematic review of the genetics involved. World J Psychiatry. 2015 Mar 22;5(1):103-11.
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstetrics and Gynecology. 2005;106(5):1071–1083.
- Whitaker RC, Orzol SM, Kahn RS. The co-occurrence of smoking and a major depressive episode among mothers 15 months after delivery. Prev Med. 2007;45(6):476–480.
- Josefsson A, Sydsjö G, Berg G, Dahl ML, Wadelius M, Nordin C. CYP2D6 genotypes and depressive symptoms during late pregnancy and postpartum. Nord J Psychiatry. 2004;58:61–64.
- Sun HS, Tsai HW, Ko HC, Chang FM, Yeh TL. Association of tryptophan hydroxylase gene polymorphism with depression, anxiety and comorbid depression and anxiety in a population-based sample of postpartum Taiwanese women. Genes Brain Behav. 2004;3:328–336
- Lin YM, Ko HC, Chang FM, Yeh TL, Sun HS. Population-specific functional variant of the TPH2 gene 2755C& gt; A polymorphism contributes risk association to major depression and anxiety in Chinese peripartum women. Arch Womens Ment Health. 2009;12:401–408.
- Fasching PA, Faschingbauer F, Goecke TW, Engel A, Häberle L, Seifert A, Voigt F, Amann M, Rebhan D, Burger P, et al. Genetic variants in the tryptophan hydroxylase 2 gene (TPH2) and depression during and after pregnancy. J Psychiatr Res. 2012;46:1109–1117.
- Sanjuan J, Martin-Santos R, Garcia-Esteve L, Carot JM, Guillamat R, Gutierrez-Zotes A, Gornemann I, Canellas F, Baca-Garcia E, Jover M, et al. Mood changes after delivery: role of the serotonin transporter gene. Br J Psychiatry. 2008;193:383–388.
- Mitchell C, Notterman D, Brooks-Gunn J, Hobcraft J, Garfinkel I, Jaeger K, Kotenko I, McLanahan S. Role of mother’s genes and environment in postpartum depression. Proc Natl Acad Sci USA. 2011;108:8189–8193.
- Mehta D, Quast C, Fasching PA, Seifert A, Voigt F, Beckmann MW, Faschingbauer F, Burger P, Ekici AB, Kornhuber J, et al. The 5-HTTLPR polymorphism modulates the influence on environmental stressors on peripartum depression symptoms. J Affect Disord. 2012;136:1192–1197.
- Doornbos B, Dijck-Brouwer DA, Kema IP, Tanke MA, van Goor SA, Muskiet FA, Korf J. The development of peripartum depressive symptoms is associated with gene polymorphisms of MAOA, 5-HTT and COMT. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1250–1254.
- Comasco E, Sylvén SM, Papadopoulos FC, Oreland L, Sundström-Poromaa I, Skalkidou A. Postpartum depressive symptoms and the BDNF Val66Met functional polymorphism: effect of season of delivery. Arch Womens Ment Health. 2011;14:453–463
- Costas J, Gratacòs M, Escaramís G, Martín-Santos R, de Diego Y, Baca-García E, Canellas F, Estivill X, Guillamat R, Guitart M, et al. Association study of 44 candidate genes with depressive and anxiety symptoms in post-partum women. J Psychiatr Res. 2010;44:717–724.
- Figueira P, Malloy-Diniz L, Campos SB, Miranda DM, Romano-Silva MA, De Marco L, Neves FS, Correa H. An association study between the Val66Met polymorphism of the BDNF gene and postpartum depression. Arch Womens Ment Health. 2010;13:285–289.
- Mileva-Seitz V, Steiner M, Atkinson L, Meaney MJ, Levitan R, Kennedy JL, Sokolowski MB, Fleming AS. Interaction between oxytocin genotypes and early experience predicts quality of mothering and postpartum mood. PLoS One. 2013;8:e61443.
- Engineer N, Darwin L, Nishigandh D, Ngianga-Bakwin K, Smith SC, Grammatopoulos DK. Association of glucocorticoid and type 1 corticotropin-releasing hormone receptors gene variants and risk for depression during pregnancy and post-partum. J Psychiatr Res. 2013;47:1166–1173.
- Moretti, M., Lato, D., Berger, H. et al. A cost-effectiveness analysis of maternal CYP2D6 genetic testing to guide treatment for postpartum pain and avert infant adverse events. Pharmacogenomics J 18, 391–397 (2018). https://doi.org/10.1038/tpj.2017.33
- Li Q, Wang S, Mei X. A single intravenous administration of a sub-anesthetic ketamine dose during the perioperative period of cesarean section for preventing postpartum depression: A meta-analysis. Psychiatry Res. 2022 Apr;310:114396. doi: 10.1016/j.psychres.2022.114396. Epub 2022 Jan 17. PMID: 35278826.
- Han Y, Li P, Miao M, Tao Y, Kang X, Zhang J. S-ketamine as an adjuvant in patient-controlled intravenous analgesia for preventing postpartum depression: a randomized controlled trial. BMC Anesthesiol. 2022 Feb 16;22(1):49. doi: 10.1186/s12871-022-01588-7. PMID: 35172727; PMCID: PMC8848809.
- Chen-Li D, Lui LMW, Rosenblat JD, Lipsitz O, Teopiz KM, Ho R, Vinberg M, Golts M, Jawad MY, Lee Y, Nasri F, Gill H, McIntyre RS. Ketamine as potential treatment for postpartum depression: A narrative review. Ann Clin Psychiatry. 2022 Nov;34(4):264-274. doi: 10.12788/acp.0082. PMID: 36282614.
- Meshkat, N. B. Rodrigues, J. Di Vincenzo, F. Ceban, S. Jaberi, R. S. McIntyre, L. M.W. Lui, J. D. Rosenblat. Pharmacogenomics of ketamine: A systematic review. Journal of Psychiatric Research Volume 145 (2022), Pages 27-34, ISSN 0022-3956, https://doi.org/10.1016/j.jpsychires.2021.11.036.
- Oates M. Suicide: the leading cause of maternal death. Br J Psychiatry. 2003;183:279–281.

