Blog

Written by Julie Bick, Ph.D.
In a previous blog, we reviewed the growing data around the role of mitochondria in biological aging and in the progression of many diseases of aging. The advancements in describing mitochondrial dysfunction have come about through novel approaches in metabolic analysis that are showing these organelles in a whole new light. This research has identified two types of mitochondrial dysfunctions- inherited mitochondrial dysfunction where deficiencies in mtDNA maintenance and function result in primary mitochondrial diseases (examples include Leigh syndrome, mitochondrial myopathy and chronic progressive external ophthalmoplegia) and then the more common pathologies such as cancer, heart failure and neurodegenerative diseases that are associated with secondary mitochondrial dysfunction.
In this blog, we look at some of the studies and techniques that have revealed the central role that mitochondria have on our cellular health, how we can maintain healthy mitochondria, and how these organelles are emerging as therapeutic targets within many areas of medicine.
Exploring Mitochondrial Function
Understanding the complex roles of mitochondria has involved many different approaches to studying their morphology and physiological function within different tissues and cell types. To help make sense of this all, the Cellular Bioenergetics of Neurodegenerative Diseases (CeBioND) Consortium are comparing data sets across different studies and disease models to help generate guidelines and standards in how mitochondrial dysfunction is monitored. This is proving to be particularly valuable regarding neurological conditions such as Parkinson’s Disease (PD), Huntington’s Disease (HD) and Alzheimer’s Disease (AD) with pathologies highly linked with mitochondrial physiology and associated specific enzyme activities. Multiple studies have highlighted specific mitochondrial dysfunctions for each of these – PD is associated with impaired respiratory chain Complex I, HD with Complexes II and III and AD with Complex IV, along with reduced α-ketoglutarate dehydrogenase activity in both PD and AD (Yin et. al. 2014). These findings back up previous studies using animal systems where drug-induced models for these diseases were generated with specific respiratory chain inhibitors (Martinez and Greenamyre 2012; Brouillet et. al. 1999) that disrupt the mitochondrial electrical gradient.
Table 1, summarizes many of the techniques used to examine mitochondria. These include methods to isolate and recover mitochondria from cells, as well as fluorescent probes that can help researchers profile the state and function of mitochondria in different cell types. The wide range of approaches is a reflection on the complex roles of mitochondria within healthy cells, and the role that mitochondrial dysfunction is now believed to play in many wide-raging diseases from cardiovascular diseases, stroke and cancer.
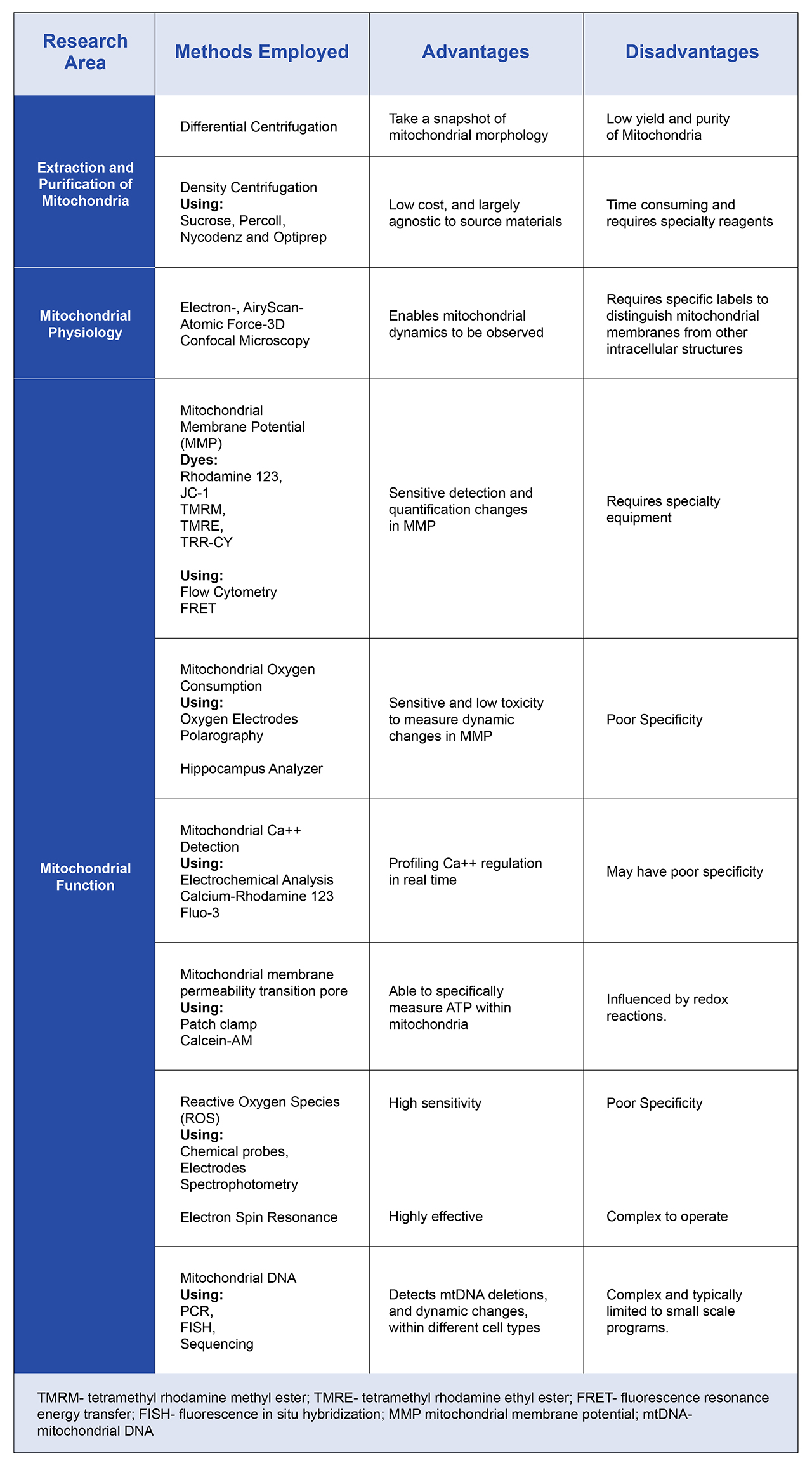
Table 1. Overview of the many diverse techniques used to profile mitochondrial function. As the roles of mitochondria are becoming increasingly complex, technologies such as these are helping researchers to understand the potential of mitochondria as therapeutic targets.
Exploring Mitochondrial Features
The mitochondrial electrochemical gradient, also known as the proton motive force (PMF), is a critical component of cellular respiration and ATP production that takes place within mitochondria. It incorporates two main factors- the membrane potential (Δψ), which is the voltage difference across the inner mitochondrial membrane, and the proton gradient (Δ𝑝𝐻), which is the difference in proton concentration (pH) across the membrane. Together, these components drive the synthesis of ATP by the enzyme ATP synthase. Because of their limited glycolytic capacity coupled with their high metabolic activity, neurons are highly dependent on the process of oxidative phosphorylation (Kann and Kovac, 2007); therefore, the measurement of the mitochondrial electrochemical gradient in the neural cells of PD and AD patients, is revealing new evidence for their central role in disease progression (Goa et. al. 2017).
There are several standardized techniques to measure the components of the mitochondrial electrochemical gradient, including fluorescent dyes that can be applied to isolated cells or tissues. These include the cationic lipophilic dyes TMRM and TMRE that accumulate in the mitochondrial matrix in proportion to the membrane potential. A high membrane potential causes increased dye accumulation, leading to a stronger fluorescence signal. A decrease in Δψ, due to proton leakage or mitochondrial damage, results in the release of the dye and a reduction in fluorescence. Similarly, Rhodamine 123 is a dye that accumulates in mitochondria based on Δψ. The intensity of fluorescence directly correlates with the mitochondrial membrane potential- a higher membrane potential results in greater dye accumulation and fluorescence.
In isolated cell systems, electrophysiological techniques can be used to determine Δψ, and track changes based on external factors; these include patch-clamp and microelectrode techniques that directly measure the electrical potential across the inner mitochondrial membrane. Although these techniques are more invasive and can be highly technically challenging, they provide direct, and quantitative measurements of the membrane potential.
The pH gradient (Δ𝑝𝐻) across the inner mitochondrial membrane can be measured using pH-sensitive dyes or specific biochemical assays. Fluorescent Dyes such as BCECF-AM (2',7'-Bis-(2-Carboxyethyl)-5-(and-6)-Carboxyfluorescein) and SNARF-1 (Seminaphthorhodafluor-1) are pH-sensitive and after they enter the mitochondrial matrix, they emit fluorescence in a pH-dependent manner. The fluorescence ratio at different wavelengths (excitation or emission) can be used to calculate the pH in the mitochondrial matrix.
Once the Δψ and Δ𝑝𝐻 are determined, the total proton motive force (PMF) can be calculated.
PMF = Δ𝜓+(𝑅𝑇𝐹)⋅Δ𝑝𝐻
where: Δψ is the membrane potential (mV), R is the gas constant, T is the absolute temperature (Kelvin), F is the Faraday constant, Δ𝑝𝐻 is the pH gradient.
The total proton motive force (PMF) is crucial for understanding the role of mitochondria in various diseases because it reflects the overall health of mitochondria and can be used to track the mitochondrial dysfunction which is the hallmark of so many diseases (Nicholls and Ferguson, 2013; Connolly et. al. 2017; Zhang and Trushina, 2017).
Maintaining Healthy Mitochondrial Flux
Mitochondrial flux is a commonly referred to and vital indicator of mitochondrial health, reflecting the ability of mitochondria to produce energy, handle metabolic substrates, maintain redox balance, and adapt to changing cellular conditions. A healthy flux indicates efficient energy production, dynamic adaptability, but significantly also incorporates effective quality control mechanisms that include the breakdown or cellular expulsion of dysfunctional mitochondria, all of which are crucial for normal cellular function.
Mitochondrial flux also influences the production and detoxification of reactive oxygen species (ROS). A normal flux ensures a balance between ROS production and scavenging, which is essential for maintaining redox homeostasis and preventing oxidative damage. Healthy mitochondria generate ROS as a by-product of electron transport, but at a controlled rate that is quickly neutralized by antioxidant defenses. This balance is critical for cell signaling and protection against oxidative stress.
Low or altered mitochondrial flux and the resulting lack of mitochondrial adaptability leads to energy deficits, decreased endurance, and poor response to physiological stressors. If mitochondrial flux is impaired, there may be excessive ROS production due to electron leakage from the ETC. Conversely, an abnormally low flux can also disrupt redox signaling. Both scenarios can lead to oxidative damage to mitochondrial DNA, lipids, and proteins, further compromising mitochondrial function and contributing to diseases like neurodegeneration, cardiovascular disorders, and aging, as well as a range of mitochondrial myopathies. In fact, in many diseases, cells exhibit altered flux rates. For example, in cancer cells, even in the presence of oxygen, there is a shift toward glycolysis ("Warburg effect") rather than oxidative phosphorylation, reflecting mitochondrial dysfunction or adaptations that favor rapid cell proliferation.
Mitochondrial flux is also closely linked to the processes of mitochondrial quality control, including mitophagy (the selective degradation of damaged mitochondria) and biogenesis (the production of new mitochondria). High Mitochondrial Flux supports effective mitochondrial turnover, where damaged or dysfunctional mitochondria are efficiently removed and replaced by healthy ones, or repair of mitochondria through a fusion process. These are both critical for maintaining a population of healthy mitochondria that can meet the cell's energy needs. In contrast to this, low or altered flux indicates impaired mitophagy or biogenesis, resulting in fragmented, or hyperfused mitochondria. The most well characterized example if this is in Parkinson's disease (Henrich et. al. 2023), in which mutations in the genes PINK1 and Parkin appear to directly disrupt the clearance of damaged mitochondria, leading to reduced mitochondrial flux, the accumulation of dysfunctional mitochondria, leading to neuronal cell death.
Other conditions associated with impaired mitochondrial network dynamics include Charcot-Marie-Tooth Disease (Schiavon et. al. 2021), optic atrophy (Lenaers et. al. 2021) and metabolic disorder or obesity (Xia et. al. 2024)
Healthy Mitochondrial flux is associated with robust mitochondrial function, where the electron transport chain is efficiently converting substrates into energy, maintaining an adequate supply of ATP for cellular processes. This is characteristic of metabolically active tissues like muscle and brain, where rapid energy production is essential. It also allows cells to adjust ATP production dynamically in response to changes in energy demand or oxygen supply. For instance, during exercise, muscle cells increase mitochondrial flux to enhance ATP production through oxidative phosphorylation, while during low oxygen conditions, cells may shift toward glycolysis. Recent research is PD is demonstrating an unexpected role for a key respiratory enzyme, PGK1 in disease symptoms, showing even modest boosts in PGK1 activity alleviate the synaptic dysfunction associated with PD (Kokotos et. al. 2024). PGK1 is expressed in the cytoplasm, but it has been shown to be translocated to the mitochondria in several diseases including PD. Within the mitochondria, PGK1 is phosphorylated resulting in the inhibition of the PDH complex resulting in inhibition of oxidative phosphorylation and the promotion of glycolysis (Li et. al. 2016).
How can we support healthy mitochondria?
Well as with many aspects of our health, a healthy diet is crucial. Consuming foods rich in essential nutrients—such as vitamins (B vitamins, vitamin C, vitamin D, and vitamin E), minerals (magnesium, zinc, selenium), and antioxidants (polyphenols, flavonoids), both supports mitochondrial function and protects them from damaging oxidative stress. Similarly, polyphenols (found in berries, green tea and dark chocolate) also provide antioxidant support, but polyphenols have also been shown to promote mitochondrial biogenesis, which has significant implications for protecting against many age-related diseases. Mitochondria-targeted antioxidants such as MitoQ™, SkQ1, and alpha-lipoic acid have been shown to reduce mitochondrial damage and improve function. CoQ10 is vital for the electron transport chain (ETC) in mitochondria and helps produce ATP. Including CoQ10-rich foods like fatty fish (e.g., salmon, mackerel), organ meats (e.g., liver, kidney), and whole grains can support mitochondrial energy production. In addition, foods high in omega-3 fatty acids (oily fish, flaxseed, walnuts and chai seeds) reduce inflammation and improve the mitochondrial membrane fluidity and integrity. Given the prokaryotic origin of mitochondria, it shouldn’t be too surprising that the gut microbiome has been shown to influence several mitochondrial functions through the production of various metabolites (Franco-Obregon et. al. 2017). These metabolites include colonic acid (CA), bile acids, ROS, pyrroloquinoline quinone, and modified fatty acids (Bajpai et. al. 2018), and they appear to influence mitochondrial function in several ways from stimulating mitochondria biogenesis, to impacting the expression of genes within mtDNA. Therefore, maintaining a healthy microbiome, through a fiber-rich diet and probiotics appears to offer many benefits to mitochondrial health.
All types of regular physical exercise from high impact cardio to resistance training and high-intensity interval training (HIIT) have been shown to improve mitochondrial health, either through stimulating mitochondrial biogenesis, increasing mitochondrial capacity or levels of electron-transport chain proteins within the mitochondria directly (Li et. al. 2022). Therefore, regular physical activity really is important as we get older not only to maintain flexibility and physical endurance, but also at the cellular level to support the cellular repair mechanisms reliant on mitochondrial-based metabolic pathways.
Interestingly, both intermittent fasting and cold exposure have been shown to promote mitophagy and mitochondrial biogenesis, resulting in the removal of damaged mitochondria and increasing the mitochondrial load of adipose tissue. As a result, both activities are associated with healthy aging or more specifically a reduction in the rate of biological aging. Interestingly, intermittent fasting has been shown to alter the gut microbiota as well as the profiles of short-chain fatty acids produced from the microbiome (Guo et. al. 2021). Several companies are now promoting intermitting fasting and probiotics with NAD+ precursor supplements such as nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN) and pyrroloquinoline quinone (PQQ) and Urolithin A (a microbial-derived gut metabolite) to specifically support healthy mitochondrial function to address aging, and early research on the effectiveness of these is largely positive (Infante, 2024. https://www.news-medical.net/health/Beyond-Energy-Mitochondrias-Role-in-Diet-and-Health.aspx; Broome et. al. 2024; Liu at al 2022)
Both alcohol and tobacco are known to increase cellular oxidative stress and damage to mitochondrial DNA, proteins and lipids and therefore should be avoided or used in moderation. I also recommend avoiding drinking out of plastic bottles- the impact of microplastics on or cells is still being determined, but like many toxins we are exposed to, the accumulation of these within our cells is likely to damage our organelles including our mitochondria (Lee et. al. 2022; Yontem et. al .al 2024), so a simple switch from plastic to glass may have a profound effect in the long run.
Lastly, as with many aspects of our health and wellbeing, sleep and stress management are important- and this is no different when it comes to mitochondrial health. Cortisol is a major generator of oxidative stress, so ensuring you get a solid 7-8 hours of sleep each night, not only reduces stress, but also provides time for your cells to undergo necessary repairs, including mitochondrial repair and biogenesis (Richardson and Mailloux, 2023).
Mitochondria as Drug Targets
Given their central role in cell biology and disease pathology, mitochondria are rapidly emerging as attractive targets for drug development. Initial mitochondrial therapies focused principally on inherited mitochondrial dysfunctional diseases (Pfeffer et. al. 2012); however, with the wave of emerging clinical evidence for the role of mitochondrial dysfunction in many other more prevalent conditions, from neurodegenerative diseases, heart failure, autoimmune diseases and cancer there is renewed interest in mitochondrial pharmacology.
In the field of oncology, engineering mitochondrial-targeted therapeutics that induce cancer cell apoptosis is a rapidly expanding area of research. The altered mitochondrial metabolism of cancer cells makes them particularly susceptible to inhibitors of glycolysis, and the hyperpolarization of the mitochondrial membrane potential may be manipulated to specifically target drugs to cancer cells, via these cancer-specific mitochondria. This strategy could be applied to new cancer drugs as well as conventional chemotherapies such as camptothecin, chlorambucil and doxorubicin that all have been demonstrated to lead to the development of resistance and undesirable side-effects traditionally. The delivery of these drugs directly to cancer-cell mitochondria could significantly improve their efficacy (Han et. al. 2014).
When it comes to new treatments for neurodegenerative diseases, mitochondria are seen as the preferred target for therapeutic intervention. Specificity to the mitochondria is being achieved in various ways such as using positively-charged lipophilic molecules, to target the mitochondrial membranes, and conjugating drugs with Szeto-Schiller (SS) peptides and other mitochondrial-penetrating peptides. When it comes to the types of drugs being assessed, these range from molecules targeting mtDNA, to specific ETC and mitochondrial enzyme inhibitors and mitophagy-promotors. Therapeutic efficacy using these approaches will depend on a better understanding of the underlying pathogenesis of these diseases and how this relates to mitochondrial health.
Final Thoughts
As with many aspects of our health, the key may lie in supporting healthy mitochondrial function as a preventative measure against disease. Research demonstrating the communication between mitochondria and other cell compartments and organelles only cement the idea that mitochondrial are more nuanced than once though. Their bi-directional communication with the microbiome has opened a new field of research exploring the role of gut microbiome with neurodegenerative conditions including PD and AD, depression and many diseases of inflammation with known associations to mitochondrial dysfunction (Jemimah et. al. 2023; Chen and Vitetta 2020; Zhang et. al. 2022).
We are only now beginning to understand how mitochondrial flux relates to diet, exercise, and the microbiome; but given the central role that these organelles play in cellular health, the concept of therapeutics that maintain or retore mitochondrial function would have positive effects on cellular homeostasis, oxidative stress management and may even offer the potential to prevent the growth of cancer cells, or the loss of neurons in neurodegenerative diseases.
References
- Yin F, Boveris A, Cadenas E. Mitochondrial energy metabolism and redox signaling in brain aging and neurodegeneration. Antioxid Redox Signal 2014;20:353–71.
- Martinez TN, Greenamyre JT. Toxin models of mitochondrial dysfunction in Parkinson’s disease. Antioxid Redox Signal 2012;16:920–34.
- Brouillet E, Conde F, Beal MF, Hantraye P. Replicating Huntington’s disease phenotype in experimental animals. Prog Neurobiol 1999;59:427–68.
- Gao J, Wang L, Liu J, Xie F, Su B, Wang X. Abnormalities of mitochondrial dynamics in neurodegenerative diseases. Antioxidants. 2017;6:25.
- Kann O, Kovacs R. Mitochondria and neuronal activity. Am J Physiol Cell Physiol 2007;292:C641–57.
- Kokotos, A., et. al. ,Phosphoglycerate kinase is a central leverage point in Parkinson’s disease–driven neuronal metabolic deficits.Sci. Adv.10,eadn6016(2024).DOI:10.1126/sciadv.adn601
- Li, X. J. et al. Mitochondria-translocated PGK1 functions as a protein kinase to coordinate glycolysis and the TCA cycle in tumorigenesis. Mol. Cell 61, 705–719 (2016)
- Nicholls DG, Ferguson S. Bioenergetics. Academic Press: London, 2013.
- Zhang L, Trushina E. Respirometry in neurons. Techniques to investigate mitochondrial funciton in neurons. Strack, Stefan, Usachev, Yuriy M (eds.), Vol. 123. Springer; 2017. p. 95–113
- Lee SE, Yi Y, Moon S, Yoon H, Park YS. Impact of Micro- and Nanoplastics on Mitochondria. Metabolites. 2022 Sep 23;12(10):897. doi: 10.3390/metabo12100897. PMID: 36295799; PMCID: PMC9612075
- Dal Yöntem F, Aydoğan Ahbab M. Mitochondria as a target of micro- and nanoplastic toxicity. Cambridge Prisms: Plastics. 2024;2:e6. doi:10.1017/plc.2024.6
- Connolly, N.M.C., Theurey, P., Adam-Vizi, V. et al. Guidelines on experimental methods to assess mitochondrial dysfunction in cellular models of neurodegenerative diseases. Cell Death Differ 25, 542–572 (2018). https://doi.org/10.1038/s41418-017-0020-4
- Broome, S.C., Whitfield, J., Karagounis, L.G. et al. Mitochondria as Nutritional Targets to Maintain Muscle Health and Physical Function During Ageing. Sports Med (2024). https://doi.org/10.1007/s40279-024-02072-7
- Liu, S. et. al. Effect of Urolithin A Supplementation on Muscle Endurance and Mitochondrial Health in Older Adults. AMA Network Open. 2022;5(1):e2144279. doi:10.1001/jamanetworkopen.2021.44279
- Franco-Obregón A, Gilbert JA. The microbiome-mitochondrion connection: common ancestries, common mechanisms, common goals. mSystems. 2017;2(3):e00018-17. doi:1128/mSystems.00018-17
- Bajpai P., Darra A., Agrawal A. Microbe-mitochondrion crosstalk and health: An emerging paradigm. Mitochondrion. 2018;39:20–25. doi: 10.1016/j.mito.2017.08.008.
- Richardson RB, Mailloux RJ. Mitochondria Need Their Sleep: Redox, Bioenergetics, and Temperature Regulation of Circadian Rhythms and the Role of Cysteine-Mediated Redox Signaling, Uncoupling Proteins, and Substrate Cycles. Antioxidants (Basel). 2023 Mar 9;12(3):674. doi: 10.3390/antiox12030674. PMID: 36978924; PMCID: PMC10045244.
- Jemimah S, Chabib CMM, Hadjileontiadis L, AlShehhi A. Gut microbiome dysbiosis in Alzheimer’s disease and mild cognitive impairment: a systematic review and meta-analysis. PLoS One. 2023;18(5):e0285346. doi:1371/journal.pone.0285346
- Chen J, Vitetta L. Mitochondria could be a potential key mediator linking the intestinal microbiota to depression. J Cell Biochem. 2020;121(1):17-24. doi:1002/jcb.29311
- Zhang Y, Zhang J, Duan L. The role of microbiota-mitochondria crosstalk in pathogenesis and therapy of intestinal diseases. Pharmacol Res. 2022;186:106530. doi:1016/j.phrs.2022.106530
- Li et. al. Exercise couples mitochondrial function with skeletal muscle fiber type via ROS-mediated epigenetic modification. Free Radical Biology and Medicine. Volume 213. 2024. Pp 409-425, ISSN 0891-5849. https://doi.org/10.1016/j.freeradbiomed.2024.01.036.
- Guo Y, Luo S, Ye Y, Yin S, Fan J, Xia M. Intermittent fasting improves cardiometabolic risk factors and alters gut microbiota in metabolic syndrome patients. J Clin Endocrinol Metab. 2021;106(1):64-79. doi:1210/clinem/dgaa644
- Henrich, M.T., Oertel, W.H., Surmeier, D.J. et al. Mitochondrial dysfunction in Parkinson’s disease – a key disease hallmark with therapeutic potential. Mol Neurodegeneration 18, 83 (2023). https://doi.org/10.1186/s13024-023-00676-7
- Lenaers, G., et. al. Dominant optic atrophy: Culprit mitochondria in the optic nerve. Progress in Retinal and Eye Research, Volume 83, 2021, 100935, ISSN 1350-9462. https://doi.org/10.1016/j.preteyeres.2020.100935
- Schiavon CR, Shadel GS and Manor U (2021) Impaired Mitochondrial Mobility in Charcot-Marie-Tooth Disease. Front. Cell Dev. Biol. 9:624823. doi: 10.3389/fcell.2021.624823
- Xia, W., Veeragandham, P., Cao, Y. et al. Obesity causes mitochondrial fragmentation and dysfunction in white adipocytes due to RalA activation. Nat Metab 6, 273–289 (2024). https://doi.org/10.1038/s42255-024-00978-0
- Pfeffer G, Majamaa K, Turnbull DM, Thorburn D, Chinnery PF. Treatment for mitochondrial disorders. Cochrane Database of Systematic Reviews 2012, Issue 4. Art. No.: CD004426. DOI: 10.1002/14651858.CD004426.pub3
- Han M., Vakili M.R., Soleymani Abyaneh H., Molavi O., Lai R., Lavasanifar A. Mitochondrial delivery of doxorubicin via triphenylphosphine modification for overcoming drug resistance in MDA-MB-435/DOX cells. Mol. Pharm. 2014;11:2640–2649. doi: 10.1021/mp500038g.

