Blog

Written by Julie Bick, Ph.D.
In recent years, the field of PGx has rapidly gained attention in the healthcare industry for its ability to enhance patient care by customizing medication protocols based individual genetic profiles. The potential of PGx testing is substantial, enabling healthcare providers to transform their approach to drug prescriptions, reduce side-effects and adverse drug reactions (ADRs), improve therapeutic efficacy, and to ultimately improve and save lives of patients. In this blog, we outline what PGx testing is, how it works, its benefits, challenges, and how it is actively contributing to patient safety today within our healthcare systems.
What is PGx testing?
PGx testing combines pharmacology and genomics to more fully understand how an individual’s genetic makeup affects their response to medications. The field emerged from the understanding that drugs are not "one-size-fits-all." Both clinical trials and traditional methods of prescribing medications often rely on population-based studies and averages, but patients can respond differently to the same drug due to their unique genetic variations; PGx profiling is helping us leverage this to provide safer therapeutic strategies to patients.
Incite Health’s PGx testing involves analyzing specific genes to predict how a person might respond to a particular medication. The test uses a simple buccal swab to collect the sample, which is then analyzed in our laboratory. The results reveal variations in genes related to drug metabolism, drug transport, and the specific drug target sites, providing insights into the patient’s potential response to a wide range of medications.
Within the human genome are various genes that encode for enzymes in the cytochrome P450 (CYP) family, and these enzymes play a critical role in drug metabolism. Variants in these genes can lead to different levels of enzyme activity, that are categorized for PGx reporting.
Poor Metabolizers (PMs): Individuals with little or no functional enzyme activity, leading to slower drug metabolism.
Intermediate Metabolizers (IMs): Those with reduced enzyme activity, resulting in slower than normal drug metabolism.
Extensive Metabolizers (EMs): Individuals with normal enzyme activity and standard drug metabolism.
Ultrarapid Metabolizers (UMs): Those with increased enzyme activity, resulting in faster drug metabolism.
These genetic differences can cause significant variations in drug responses among individuals. For example, a poor metabolizer might experience toxicity at standard drug dosages because they are not processing it at the optimal rate, whereas an ultrarapid metabolizer may require higher doses to achieve therapeutic effects because he or she is breaking the drug down too quickly. Therefore, knowing the metabolizer profile by analyzing these CYP genes enables healthcare providers to tailor both drug selection and their dosages for optimal safety and efficacy.
The nomenclature for gene variant profiling through PGx is unique; the PGx report provides descriptions of each of the two copies of the gene- each haplotype is named by the gene name followed by a major star (*) allele and subvariant assignment. The *1 assignment is typically the default or reference allele, with other * alleles named as they are described. For example, a PGx profile of CYP2C19 *17/*17 means this individual carries two *17 functional alleles for the CYP2C19 gene. However, in some cases an individual may have more than two copies of a gene- this is especially common for the gene CYP2D6. So, a person may see CYP2D6 *1/*2x2 on their PGx report. This means that this person carries 1 copy of the *1 allele and 2 copies of the *2 allele. The Incite Health PGx testing includes copy number variation analysis for CYP2D6 since this is a critical feature for some drug metabolizing profiles.
In some instances, the specific DNA variant will be more thoroughly described; for example, the CYP2D6*2 allele actually contains two variants – c.2851C>T and c.4181G>C. In this example the number represents the location of nucleotide of the CYP2D6 gene that is changed followed by a description of the nucleotide substitution. This may also include a description of the respective amino acid change and its location. In the example above, the full description would be: CYP2D6 *2 rs16947 (c.2851 C>T) (p.R296C) and rs1135840 (c.4181G>C) (pS486T).
How this simple test is saving lives
PGx testing represents a simple tool to save lives in several key areas, one of the most profound of which is by flagging patients for risk of adverse drug reactions (ADRs) before they receive the drug. ADRs are among the leading causes of morbidity and mortality in healthcare systems worldwide. In the United States alone, ADRs account for over 100,000 deaths annually and are responsible for a significant number of hospital admissions (Lazarou et. al. 1998; Gurwitz et. al. 2000). Many ADRs are caused by variations in drug metabolism, which can lead to drug toxicity &/or therapeutic failure.
Several drug-specific adverse reactions have been described, across a range of medication types from oncology treatments to cardiovascular drugs and neuropsychiatric therapeutics, and many of these have been linked to genetic variants that can be readily profiled using PGx testing (Phillips et. al. 2001)
Optimizing Cardiovascular Therapy
Cardiovascular diseases, including heart attacks, strokes, and hypertension, are among the leading causes of death worldwide, and with our increasingly poor diets and sedentary lifestyles, it seems likely that this will not change anytime soon. PGx testing is proving to be invaluable in optimizing cardiovascular therapies, particularly in the use of anticoagulants, antiplatelet drugs, and statins to lower cholesterol.
A well-documented and recognized example of this is the drug warfarin (brand name Coumadin), a commonly prescribed anticoagulant that acts by inhibiting the synthesis of vitamin K-dependent clotting factors. Warfarin has a narrow therapeutic range resulting in a high risk of bleeding complications; therefore, the dose used must be carefully tailored according to the patient’s response and this is measured as INR (International Normalized Ratio). The dose of warfarin required to achieve target anticoagulation is influenced by clinical and lifestyle factors (age, weight, gender, smoking status and other medications taken by the patient) as well as key genetic factors. Variants in genes such as CYP2C9 and VKORC1 significantly affect how patients metabolize warfarin and their sensitivity to its effects. The CYP2C9 gene is highly polymorphic with over 60 *alleles described. CYP2C9 influences how quickly warfarin is metabolized and specific variants CYP2C9*2 and *3 are associated with lower dose requirements to reach target INR because they represent low or intermediate warfarin metabolism profiles. In contrast, CYP2C9*1/*2 genotype requires no dose adjustment. A dose reduction of warfarin is also recommended for individuals who carry two copies of the VKORC1 variant VKORC1 c.1639G>A/A. In 2017, the FDA updated the drug label for warfarin to include simple guidelines to incorporate PGx testing into both warfarin dosing and the timing of dose increases (see below Table 1).
Warfarin Therapy and VKORC1 and CYP Genotype - Medical Genetics Summaries - NCBI Bookshelf (nih.gov)
Table 1. A. Summary of PGx Recommendations for Warfarin Dosing and B. Dose Escalation
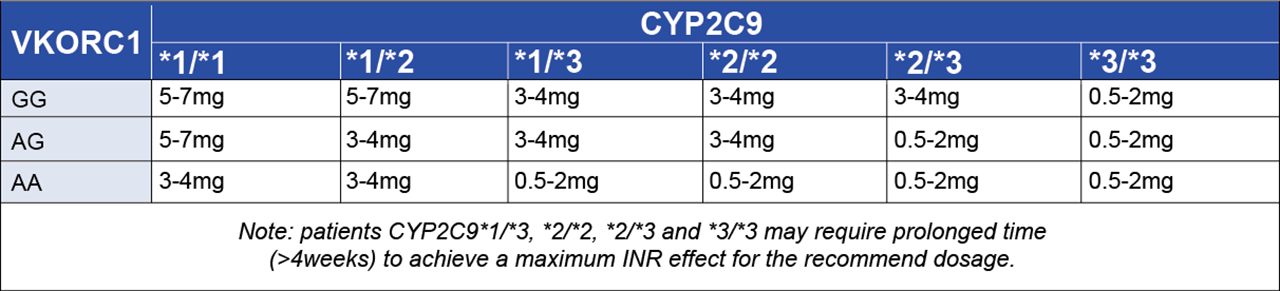
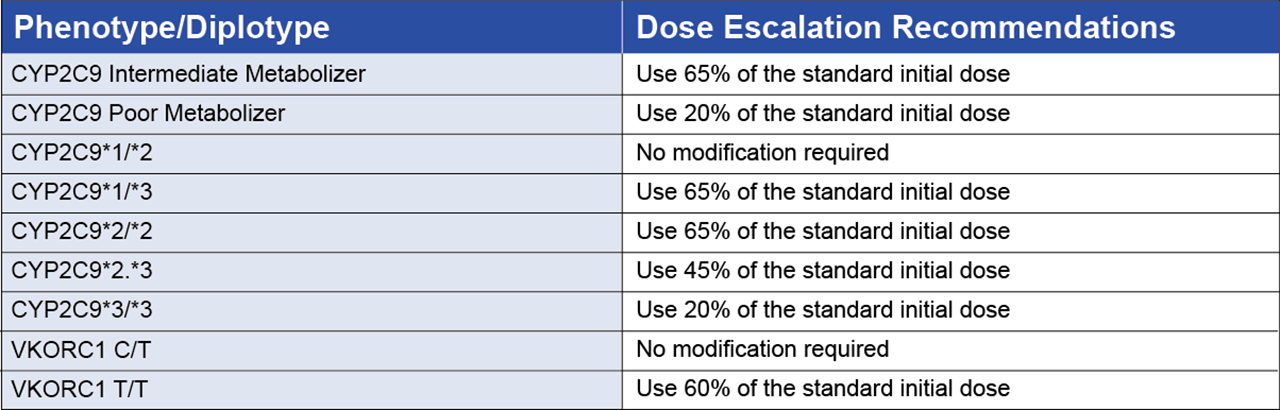
By incorporating these guidelines into the standard of care, PGx testing is being used to identify these genetic variants in patients, allowing for more accurate dosing, reducing the risk of severe bleeding events, and ultimately saving lives.
As with warfarin, clopidogrel (Plavix), an antiplatelet medication used to prevent blood clots in patients with coronary artery disease. Clopidogrel is activated in the liver by the enzyme CYP2C19. However, patients with certain CYP2C19 variants (CYP2C19*2 and CYP2C19*3) are "poor metabolizers," meaning they do not effectively convert clopidogrel to its active form, resulting in reduced drug efficacy and an increased risk of adverse cardiovascular events. Conversely, CYP2C19*17 exhibits increased enzyme activity resulting in increased platelet inhibition of clopidogrel (Tirock, et. al. 2010). PGx profiling of the CYP2C19 variants can identify these poor and rapid metabolizers, enabling the use of alternative antiplatelet therapies, such as ticagrelor (Brilinta) or prasugrel (Effient), that do not require CYP2C19 for activation.
The Value of HLA-Profiling by PGx
Another example of how PGx testing is saving lives is with the use of abacavir for HIV treatment. Abacavir is an inhibitor of a reverse transcriptase enzyme used by the AIDS virus for replication. The drug is used as part of a multi-drug, highly active retroviral therapy (HAART). However, some patients receiving the drug experienced life-threatening hypersensitivity reactions to abacavir. A 2007 presentation of two clinical studies at an AIDS conference in Australia showcased evidence for PGx markers for this abacavir hypersensitivity. These studies showed that individuals with the HLA-B*57:01 allele are at high risk for a severe and potentially fatal hypersensitivity reaction to abacavir. Within weeks, there was an increase in PGx testing for this allele, which at the time was identified in 5-8% of patients initiating abacavir therapy (Lai-Goldman and Faruki, 2008). By mid 2008 the FDA had updated the drug labeling for abacavir to include HLA-B PGx profiling. This demonstrates the potential of pivotal clinical studies that incorporate simple PGx testing to impact drug safety and prevent life-threatening adverse drug reactions.
Although the mechanism for this association is not fully understood, particular HLA alleles are associated with an increased risk of other serious drug toxicities including toxic epidermal necrolysis (TEN) and Stevens-Johnson Syndrome (SJS) which are collectively often referred to as severe cutaneous adverse reactions (SCAR) (Hung et. al. 2005, Chung et. al. 2004). Within European populations the allele HLA-B*5801 has been associated with allopurinol-induced SCAR, whereas East Asian populations display highest frequencies of allele HLA-B*1502 associated with carbamazepine-induced SJS. SCAR can be devastating, and with no approved therapeutic protocols, the mortality rates are high at around 30%. Prophylactic PGx testing that incorporates HLA is one of the few ways that SCAR can be addressed, and there is a significant push for more wide-spread adoption of PGx testing to help avoid the occurrence of these reactions altogether.
Precision Oncology
Another field of medicine where PGx is really providing great promise is with cancer treatments. Traditional chemotherapy drugs often come with severe side effects, and their effectiveness can vary greatly between patients. PGx testing enables oncologists to tailor cancer therapies based on the genetic profile of both the patient and the tumor. One of the best described successes of this is in breast cancer patients with HER2-positive tumors benefit from targeted therapy with trastuzumab (Herceptin), a monoclonal antibody that specifically targets HER2 receptors on cancer cells. PGx testing can identify patients who will benefit from this treatment, helping to manage the cost of treatment by providing this expensive drug only to patients who will benefit, and sparing others from unnecessary side effects. Collectively, this optimizes therapeutic outcomes and enables cancer patients to live longer and more fulfilling lives.
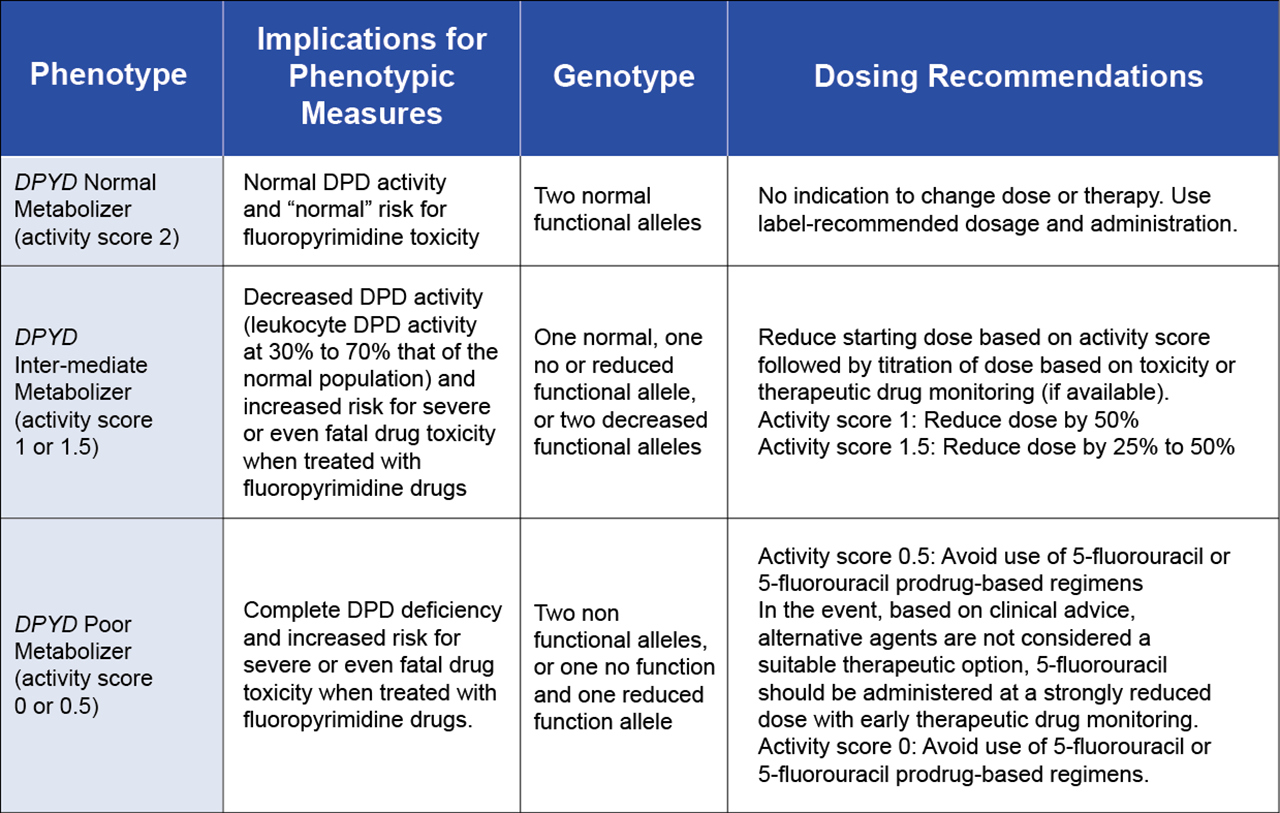
Another example of PGx testing to support oncology is in the profiling of DPYD gene variants can predict toxicity risk to the fluoropyrimidine drugs 5-fluorouracil (5-FU) and capecitabine, two commonly used chemotherapeutic agents. Patients with certain DPYD variants have a reduced ability to metabolize these drugs, leading to severe toxicity and frequently death. The genetic basis for this toxicity was first published in 1988 (Diasio et. al. 1988), however, the associated PGx guidelines were only published in 2013 by Clinical Pharmacogenetics Implementation Consortium (CPIC), with the latest update in 2017.
https://cpicpgx.org/guidelines/guideline-for-fluoropyrimidines-and-dpyd/
5-fluorouracil has a very narrow therapeutic window, meaning there is only a small difference between the dose for minimum efficacy and maximum tolerable dose. In fact, only 1-3% of the administered drug is metabolized to the active cytotoxic form, with the remainder excreted in the urine. The enzyme DPD is the rate-limiting step in the conversion of 5-fluorouracil to dihydrofluorouracil and variations in this enzyme activity are critical to effective treatment. Reduced DPD activity leads to reduced drug clearance and dose-related toxicities (Diasio et. al. 1988). This increased risk of toxicity is associated with DPYD*2A and DPYD*13, whereas as the alleles, described as c.2846A>T and c.1129-5923C>G result in moderately reduced DPD activity and patients with these alleles should be flagged for increased surveillance and modified treatment protocols (see Table 2). Decreased DPYD Identifying these patients before treatment with this simple PGx test allows for dose adjustments or the selection of alternative therapies, preventing potentially fatal side effects. Currently, even though the European Medicines Agency (EMA) has issued recommendations and almost everyone in the US healthcare industry agrees that DPYD PGx testing before starting fluoropyrimidine chemotherapy is the right things to do for patients, the FDA has yet to issue requirements; and as such, it is the pharmacists who are leading the efforts to safeguard these patients.
Table 2. Recommended Dosing of fluoropyrimidines by DPD phenotype and DPYD Genotype
Managing Pain with Greater Precision
Pain management, particularly in the context of opioid prescriptions, is another area where pharmacogenomic testing can save lives. The opioid epidemic has highlighted the risks associated with opioid misuse, addiction, and overdose. Although opioid prescriptions have fallen from their 2010-2012 peak, the so-called morphine milligram equivalents of opioids prescribed per person are now three times what they were 20 years ago. IT is estimated that even today 21-30% of patients who are prescribed opioids for chronic pain misuse them, with between 8-12% developing an opioid use disorder. (https://www.cdc.gov/drugoverdose/data/prescribing/prescribing-practices.html)
(https://www.drugabuse.gov/drug-topics/opioids/opioid-overdose-crisis)
Genetic testing can help identify patients who are more susceptible to addiction or who may require alternative pain management strategies. The enzyme CYP2D6 is crucial in metabolizing many opioids, such as codeine, hydrocodone and tramadol, into their active forms. Poor metabolizers may not receive adequate pain relief, leading to potential overdose from increasing doses, while ultrarapid metabolizers might convert these drugs too quickly, resulting in dangerous levels of active metabolites within their blood streams, and a higher risk of overdose. PGx testing is being used to guide the selection of safer, more effective pain management options, reducing the risk of overdose and improving patient outcomes (Bright et. al. 2021).
The Future of PGx Testing For Safer Therapeutic Interventions.
With ongoing research and technological advancements poised to expand the utility and accessibility of PGx testing, there are other factors that will determine how well this is integrated into best practice. These include the integration PGx data into electronic health records (EHRs) to enable healthcare providers to make informed decisions at the point of care, coupled with tools such as automated alerts and clinical decision support recommendations to enhance the selection of appropriate medications and dosages. All of this will require increased recognition of the benefits of PGx to drive policies and regulations. This could include standardized guidelines for the use of PGx testing in clinical practice and broader insurance coverage for these tests.
References
- Lazarou J, Pomeranz B, Corey PN. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA 1998;279:1200–1205.
- Gurwitz JH, Field TS, Avorn J, McCormick D, Jain S, Eckler M, et al. Incidence and preventability of adverse drug events in nursing homes. Am J Med 2000;109(2):87–94.
- Philips KA, Veenstra DL, Oren E, Lee JK, Sadee W. Potential role of pharmacogenomics in reducing adverse drug reactions. JAMA. 2001;286:2270–9
- Lai-Goldman, M., Faruki, H. Abacavir hypersensitivity: a model system for pharmacogenetic test adoption. Genet Med 10, 874–878 (2008). https://doi.org/10.1097/GIM.0b013e31818de71c
- Hung SI, Chung WH, Liou LB, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005;102:4134–4139.
- Chung WH, Hung SI, Hong HS, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428:486.
- Tiroch KA, Sibbing D, Koch W, Roosen-Runge T, Mehilli J, Schömig A, et al. Protective effect of the CYP2C19 *17 polymorphism with increased activation of clopidogrel on cardiovascular events. Am Heart J. 2010;160(3):506–12
- Diasio RB, Beavers TL, Carpenter JT. Familial deficiency of dihydropyrimidine dehydrogenase. Biochemical basis for familial pyrimidinemia and severe 5-fluorouracil-induced toxicity. J Clin Invest. 1988 Jan;81(1):47-51. doi: 10.1172/JCI113308. PMID: 3335642; PMCID: PMC442471.
- Bright DR, Petry N, Roath E, Gibb T. Engaging pharmacogenomics in pain management and opioid selection. Pharmacogenomics. 2021 Sep;22(14):927-937. doi: 10.2217/pgs-2021-0044. Epub 2021 Sep 15. PMID: 34521258; PMCID: PMC8656342.

