Blog
Written by Julie Bick, Ph.D.
Any immunologist will tell you that the immune system is a highly complex network of communication between a diverse array of different cell types. Far from being static, the immune system is in a constant state of flux and responding to the many external challenges and to the homeostatic mechanisms that control it. At the center of this all are cytokines, hormonal messengers that regulate most of the biology of the immune system.
T-cells are the main source of these chemical messengers, which come in two ‘flavors’ pro-inflammatory and anti-inflammatory, and collectively cytokines orchestrate the functions of the immune cells through a series of transcription factors, epigenetic changes including DNA methylation and cell signaling pathways (Kaiko et. al. 2008). In this blog, we explore the different branches of the T-cell-based immune responses, how imbalances in these can lead to inflammatory-based diseases, and importantly, how balance can be restored.
T Cell Profiles
T lymphocytes expressing CD4 are known as helper T cells, and these are the most prolific producers of cytokines. CD4 T-cells are subdivided into several subtypes including Th1, and Th2, and the cytokines that they produce are referred to as Th1- type and Th2-type respectively. There is also the subtype, Th17 that is associated with the production of the cytokine IL-17, which alongside Tregs cells acts to dampen down the Th1 and Th2 opposing functions. We need these different T-cell subtypes to fulfill the many activities associated with the defense of the body against various pathogens and cancer cells (Lucey et. al. 1996).
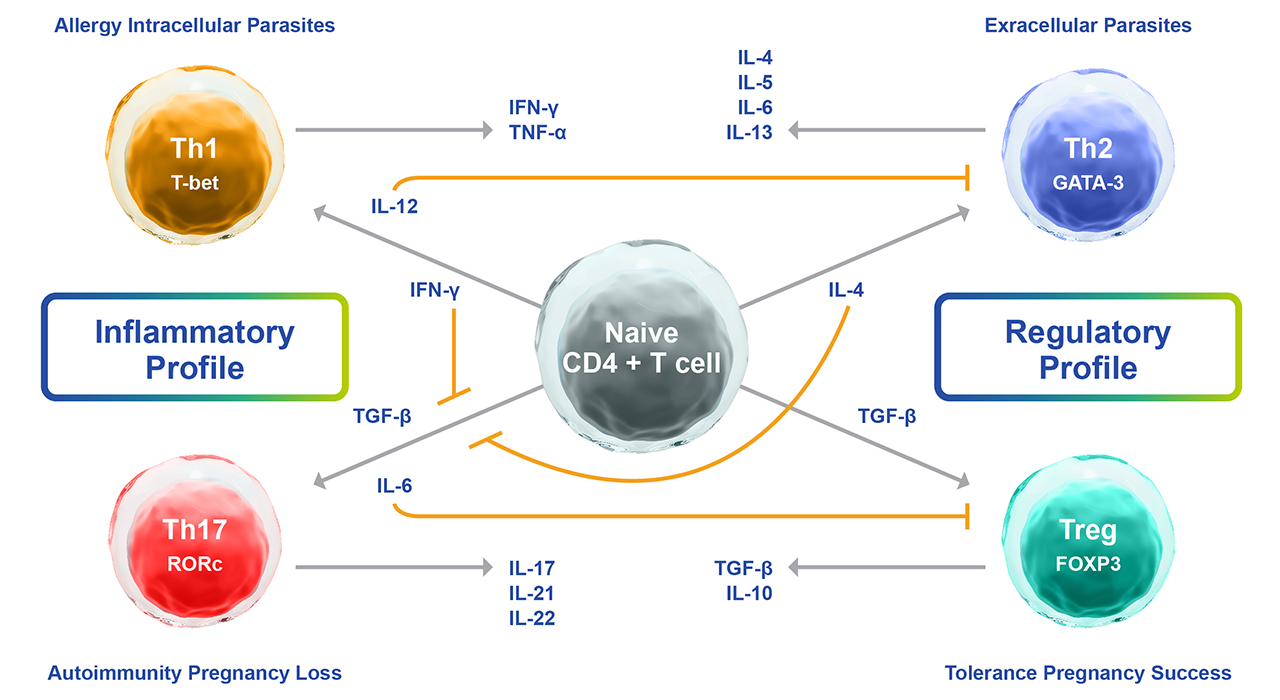
Fig. 1. Summary of the cytokine-driven mechanisms of naïve T-cell differentiation into Th1, Th2, Th17, or Tregs.
Th1 Profile
In general, Th1 cells are thought to be associated with fighting intracellular infections such as viruses and intracellular bacteria as well as cancer cells in the process called cell-mediated immunity. Th1 responses are typically upregulated during acute infection and downregulated by chronic conditions and autoimmunity.
The path to generating these Th1 ‘warrior’ infection-fighting cells begins at the site of the infection where macrophages and dendritic cells secrete IL-12, IL-2, and type 1 IFNs (IFN-α and IFN-β) and migrate to a lymph node to present the foreign antigen to naïve CD4 T-cells. Recognition of viral or microbial antigens results in the release of more IL-12, stimulating the conversion of antigen-specific CD4 naïve T-cells into programmed Th1 cells. The IL-12 also activates natural killer cells to release IFN-γ. The programmed Th1 cells start to release their own Th1 effector cytokine IFN-γ which in turn activates macrophages that then stimulate the production of antibodies by B-cells and activates other macrophages to phagocytize the infected cells. This process includes the engagement of complement proteins that enhance phagocytosis through a process called opsonization. Under normal conditions, all of these activities are regulated through this functional IFN-γ mediated activation loop that works to stimulate surrounding naïve T-cells to establish a polarized response toward a Th1 phenotype. This in turn acts to maintain the fight against the infection until it is cleared.
Th2 Profile
In contrast to Th1 cells fighting intracellular infections, Th2 cells potentiate a humoral response and are involved in fighting extracellular parasitic infections that are too large to be phagocytized by macrophages. During parasitic infections, macrophages release IL-4 which is central in the conversion of naïve CD4 T-cells to produce Th2 cells. These mediate the production of increased IL-4, IL-5, IL-9, IL-10, and IL-13 that act to stimulate B-cells to produce IgE antibodies targeting the parasite, activate mast cells and eosinophils and increase the production of mucus in the digestive tract respectively. Collectively, these activities work to label the parasite for destruction and elimination from the body. IL-6 release also inhibits IFN-γ production, and this contributes to the establishment of the Th2 biased.
In 2008, Veldhoen et. al. described the transition of Th2 cells into a subset now referred to as Th9 cells, characterized by their secretion of IL-9. It is now known that Th9 cells can be induced from naïve T-cells by IL-4 and TGF-β, or Th2 cells from TGF-β alone. Their roles have yet to be fully understood, but they are thought to play a role in fighting helminthic infections, as well as autoimmunity and allergy development (Kaplan, M., 2013).
Th17 Profile
Th17 T-cells are named after the signature cytokine that they generate, IL-17 (Harrington et. al. 2005). These cells are involved in tackling microbes outside of the cell, including fungal infections, particularly at mucosal and epithelial barriers (Khader, et. al. 2009). In this instance, CD4 naïve T cells are converted by IL-6 and TGF-β to Th17 cells, which produce IL-17, IL-17F, IL-6, IL-22, and TNF-α to recruit neutrophils to the site of the infection and stimulate fibroblasts, macrophages, and endothelial cells to elicit inflammatory mediator and chemokine release to create a state of inflammation and stimulate epithelial cells to produce specific peptides with antimicrobial properties.
Th17 cells and the cytokines that they secrete are generally considered to be pro-inflammatory and have therefore been implicated in many autoimmune diseases including type 1 diabetes, rheumatoid arthritis, asthma, IBD, multiple sclerosis, and systemic lupus erythematosus (Guery and Hugues, 2015).
Unlike Th1 and Th2, Th17 cells display significant plasticity, with studies showing their conversion towards Th1 in individuals with autoimmune diseases, cancer, or chronic infections, and towards Th2 in patients with asthma (Lee, et al 2009). Th17 cells have also been shown to acquire immunosuppressive functions by converting towards Treg FoxP3+ phenotypes, particularly in the backdrop of autoimmune diseases or chronic infections.
The Role of Tregs
Treg cells, short for regulatory T cells, are a specialized subset of T cells that play a crucial role in maintaining immune homeostasis and preventing excessive immune responses that could lead to autoimmunity and inflammation. They are typically identified by their expression of the transcription factor FoxP3 (Forkhead box P3), the master regulator of Treg functions (Workman et. al. 2010). Tregs can be generated in the thymus during T cell development or induced from conventional CD4+ T cells in the periphery through the action of TGF-β or Treg-specific antigens.
The primary role of Treg cells is to suppress immune responses and maintain immune tolerance by inhibiting the activation and proliferation of other immune cells, such as effector T cells and antigen-presenting cells (APCs), thereby preventing excessive immune reactions and tissue damage. They have been found in lymphoid organs, peripheral tissues, and localized sites of inflammation, where they express high levels of the interleukin-2 receptor (IL-2R) alpha chain (CD25). IL-2 is an essential cytokine for Treg cell development and maintenance, and IL-2 signaling is key to their survival and suppressive functions. They also produce TGF-β to induce the generation of more Treg cells and IL-10 to inhibit macrophage function.
In addition to CD4 and CD25, Treg cells express other cell surface markers, such as CD127 (IL-7 receptor alpha chain, a marker often downregulated on Treg cells), CTLA-4 (cytotoxic T-lymphocyte-associated protein 4), and GITR (glucocorticoid-induced tumor necrosis factor receptor-related protein), which are involved in their regulatory function. Tregs can exert their suppressive effects in both an antigen-specific and non-specific manner through interaction with APCs or effector T cells respectively.
Treg cells play a critical role in immune regulation and immune system balance, ensuring that the immune response is appropriately targeted and controlled. Their unique features and functions make them essential players in maintaining overall health and preventing immune-mediated diseases. However, dysfunction or deficiency of Treg cells can lead to loss of immune tolerance, contributing to autoimmune diseases, allergies, and other immune-related disorders.
T Follicular Helper Cells
T follicular helper cells (Tfh cells) are a specialized subset of CD4+ T cells that play a crucial role in regulating the adaptive immune response in the germinal centers of secondary lymphoid organs where they are primarily involved in promoting B cell activation, proliferation, and differentiation, which are essential steps in the generation of effective antibody responses. Tfh cells directly interact with B cells that have encountered antigens from pathogens or vaccines, and after recognizing specific antigens presented on the surface of B cells, Tfh cells provide essential signals that initiate B cell activation. This includes the production of co-stimulatory molecules and cytokines mainly IL-21 and IL-4, that are critical for the survival, proliferation, and differentiation of B cells into plasma cells that produce antibodies to fight the infection. Soon after initial infection, Tfh cells support the process of affinity maturation during which B cells undergo somatic hypermutation, leading to the generation of B cells with higher affinity for the antigen, and helping to select B cells with the most effective antigen receptors. As the infection-fighting process progresses, Tfh cells also induce B cells to undergo class switching, a process where B cells change the type of antibody they produce. This allows B cells to produce different classes of antibodies, such as IgM, IgG, IgA, and IgE, with varying functions to combat diverse pathogens throughout the body.
Tfh cells contribute to the generation of memory B cells that "remember" previously encountered antigens and enable a faster and more robust immune response upon re-exposure to the same pathogen.
Patients with low Tfh cell levels may experience various effects on their immune system functions, leading to weakened immune responses against infections, and less efficient clearing of pathogens.
This also extends to the efficacy of vaccines which rely on the generation of memory B cells and long-lasting antibody responses. Low Tfh cell levels may hinder the development of robust memory B cell populations, leading to a suboptimal response to vaccines and reduced protection against vaccine-preventable diseases.
Since Tfh cells have regulatory functions to prevent the generation of self-reactive antibodies and maintain immune tolerance, reduced Tfh cell activity is associated with an increased risk of autoimmunity. Lastly, low Tfh cell levels may lead to difficulty in controlling chronic infections, allowing the pathogens to persist and cause ongoing health issues.
In contrast, high Tfh cell levels may play a role in the development of autoimmune diseases and exacerbate chronic inflammation and allergies. Tfh cells are increasingly recognized for their role in antitumor immune responses. High Tfh cell numbers may correlate with better immune surveillance against certain cancers, leading to increased infiltration of T cells into tumor sites and potentially improved outcomes in cancer patients.
T Follicular Regulatory Cells
T follicular regulatory cells (Tfr cells) are a specialized subset of regulatory T cells (Tregs) that play a critical role in regulating the immune response within the germinal centers of secondary lymphoid organs, such as the lymph nodes and spleen. Germinal centers are specialized regions where B cells undergo intense proliferation, somatic hypermutation, and affinity maturation to produce high-affinity antibodies during an immune response. Tfr cells help control the activation and proliferation of B cells within the germinal center, but they also function to limit the excessive expansion of B cells and prevent the formation of autoantibodies and unwanted immune responses. During infection, Tfr cells aid in the process of affinity maturation, which involves the selection of B cells with the highest affinity for the antigen. This helps ensure that the immune system produces more effective antibodies to combat pathogens. However, these same Tfr cells also play a role in immune tolerance by preventing the generation of harmful self-reactive antibodies that could lead to autoimmune diseases. Tfr cells can suppress the function of other immune cells, such as T follicular helper cells (Tfh cells), which are responsible for providing signals to B cells and promoting their differentiation into plasma cells.
The balance between Tfh cells and Tfr cells is crucial for the proper functioning of the immune response. Too much Tfh activity without adequate Tfr regulation could lead to uncontrolled B cell responses, while excessive Tfr activity may impair the generation of protective antibodies against pathogens. Individuals with high levels of Thr cells have a higher risk of excessive suppression of Tfh and B cell activity within the germinal centers. While this may help prevent autoimmunity and limit the generation of unwanted or self-reactive antibodies, it can also dampen the immune response to infections and impair the production of protective antibodies, making the individual more susceptible to infections. Excessive Tfr cell activity might contribute to an overall immunosuppressive state, affecting not only the antibody response but also the activity of other immune cells involved in fighting infections and tumors.
High levels of Thr cells may result in uncontrolled B cell activation and excessive production of antibodies that can lead to autoimmunity.
T follicular regulatory cells play an essential role in orchestrating the adaptive immune response, ensuring that B cells produce effective antibodies while maintaining self-tolerance and preventing autoimmunity. Their study is an active area of research, and understanding their mechanisms could have implications for vaccine development, autoimmune diseases, and other immune-related conditions (Fu et. al. 2018).
Balancing the Immune System
Th1, Th2, and Th17 populations and the effector cytokines that they release are antagonistic to each other and so in response to a particular infection, one response will dominate until the infection is cleared. The balance of Th1 versus Th2 functions is important and Th17 and Tregs work together to restore balance and maintain a well-functioning immune system. Dysregulation or imbalance of Th1, Th2, and Th17 responses can contribute to chronic inflammation, tissue destruction, and the progression of chronic diseases. Immunomodulatory therapies that target these T helper cell subsets have shown promise in managing and treating certain chronic diseases by modulating the immune response and restoring immune balance. However, the specific roles of these T helper cell subsets can vary depending on the disease and the stage of disease progression, making therapeutic targeting complex and context-dependent.
Th1 Dominance
When Th1 cytokines are dominating, individuals are at risk of developing organ-specific autoimmunity, such as those commonly associated with Multiple Sclerosis, Hashimoto’s, and Type 1 diabetes. Th1 dominance is caused by the overstimulation of T cells, NK cells, and macrophages, and the associated elevated IFN-γ inhibits the production of most IgG and IgE antibodies but increases the secretion of IgM antibodies. The symptoms of Th1 dominance include fatigue, decreased appetite, and age-related health problems including insomnia, depression, and cognitive impairment. A Th1 dominant state is considered inflammatory, and therefore anti-inflammatory strategies are adopted to restore balance these include a diet rich in omega-3 fatty acids, vitamin D, curcumin, epigallocatechin gallate (found in green tea), quercetin, resveratrol, and both ginger and garlic, coupled with herbal supplements such as licorice root and ashwagandha and with medicinal mushrooms (Reishi, shiitake and maitake). Individuals who have developed chronic conditions driven by Th1 functions may benefit from a range of therapeutic drugs that have been shown to improve symptoms. These include general inflammatory drugs such as corticosteroids, immunosuppressants (azathioprine, mycophenolate mofetil, methotrexate) and calcineurin inhibitors, as well as more targeted approaches such as TNF-α inhibitors (adalimumab, infliximab, etanercept), JAK inhibitors (tofacitinib, baricitinib), IL-12/IL-23 inhibitors (ustekinumab), anti-IL2 receptors (daclizumab, basiliximab), and anti-IFN-γ antibodies (fontolizumab).
Th2 Dominance
Individuals with Th2 dominance are at higher risk of food allergies, mast cell disorders, eosinophilic disorders, and autoimmune diseases. Th2 dominance is defined by higher levels of IL-4, IL-5, and IL-13, resulting in increased and often chronic inflammation. Several factors can contribute to Th2 dominance, including genetic predisposition, environmental triggers (such as exposure to allergens), and dysregulation of immune signaling pathways. As with Th1 dominance, foods rich in omega-3 fatty acids, vitamin D, curcumin, and quercetin have all been shown to help restore balance. In addition, vitamin C and butterbur extract have been shown to help reduce Th2-related inflammation, and certain probiotic strains such as Lactobacillus rhamnosus and Bifidobacterium lactis have also been shown to reduce Th2-related inflammation.
As with Th1 dominance, anti-inflammatory drugs including corticosteroids can help manage symptoms and restore balance. In addition, anti-IL-4 and anti-IL-13 antibodies such as dupilumab have been shown to improve conditions such as atopic dermatitis and asthma associated with Th2 cytokines IL-4 and IL-13. Similarly, severe eosinophilic asthma is associated with increased levels of IL-5 that can be managed with the anti-IL-5 antibodies mepolizumab and reslizumab. Th2-related allergic responses such as allergic rhinitis can be managed with leukotriene modifiers such as montelukast and zarirlukast. Lastly, cromolyn sodium stabilizes mast cells and inhibits the release of inflammatory mediators including those associated with Th2 responses. Similar therapeutic strategies are being employed to address a range of mast cell disorders and other allergic conditions.
Th17 Dominance
Th17 dominance refers to an imbalance in the immune system's T helper cell responses, where the Th17 subset of T helper cells becomes overactive or predominant compared to other subsets, particularly Th1 and Th2 cells. Th17 cells are characterized by their production of the cytokines IL-17A, IL-17F, IL-22, and IL-21. They play a crucial role in orchestrating the immune response against certain bacterial and fungal infections but are also involved in promoting inflammation and tissue repair. However, in Th17 dominance, the Th17 response becomes excessive, leading to an overactive or exaggerated Th17-mediated immune response and increased inflammation. This can contribute to the development of various autoimmune and inflammatory conditions. Several factors can contribute to Th17 dominance, including genetic predisposition, dysregulation of immune signaling pathways, and environmental triggers.
Unique Immune System Responses to Pregnancy
During pregnancy, there is a delicate balance between the mother's immune system and the fetus. The maternal immune system must tolerate the presence of the semi-allogeneic fetus (since it carries antigens from the father, which are foreign to the mother's immune system) while still protecting against infections. Some studies have suggested that there may be an increase in Th17 cells during certain complications of pregnancy, such as pre-eclampsia, which is characterized by high blood pressure and damage to organs like the liver and kidneys. Th17 cells have been implicated in promoting inflammation, and an imbalance in Th17-related responses might be involved in the pathogenesis of pre-eclampsia. It is important to consider that the immune system responses during pregnancy are highly complex and involve various factors including Tregs which help suppress immune responses and maintain tolerance to the fetus.
Achieving Overall Immune System Balance
A well-balanced diet with anti-inflammatory foods, such as fruits, vegetables, whole grains, and healthy fats, may help support overall immune health and the inclusion of curcumin and omega-3 fatty acids provide anti-inflammatory effects. Therapeutic drugs including nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, and disease-modifying antirheumatic drugs such as sulfasalazine and methotrexate can help manage Th17-driven inflammation. In addition, Janus Kinase (JAK) inhibitors, such as tofacitinib and baricitinib, have been used to help manage Th17-related inflammation in conditions such as rheumatoid arthritis, and anti-IL-17a antibodies including secukinumab, ixekizumab, and brodalumab have been used to manage Th17-driven inflammation for conditions such as psoriasis and psoriatic arthritis.
Profiling T-Helper Responses Using Flow Cytometry
Flow Cytometry is the perfect platform for determining an individual’s T-cell profile since it can be used to both detect and enumerate the different T-cell subtypes. There are many versions of flow cytometry panels and gating strategies that can be established to identify T-cell subtypes, either using surface staining, or a combination of surface and intracellular staining. An example is shown below. This is a panel offered by Beckton Dickenson that identifies subsets of T helper cells using a CD4 T-cell subset panel.
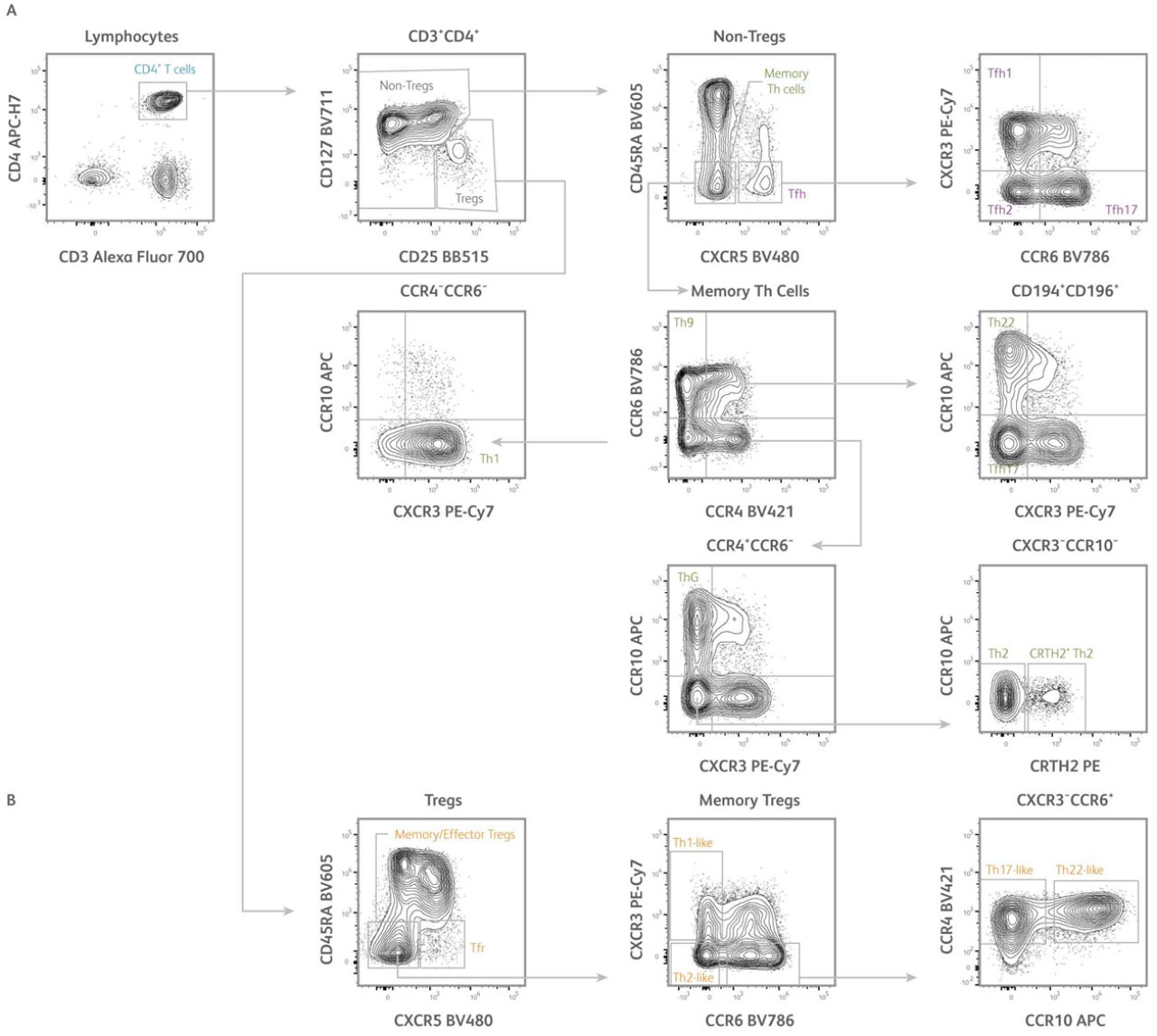
Fig. 2. Identification of subsets of human T helper cells using the CD4+ T cell subset panel. Representative analysis of PBMCs isolated from human healthy subjects. T cells were first gated based on scatter properties typical of lymphocytes (not shown).
A) After gating on CD3⁺CD4⁺ T cells, regulatory T cells (Tregs) and conventional T cells (non-Tregs) could be identified based on differential expression of CD127 and CD25. From the non-Tregs gate, conventional memory Th cells and T follicular helper cells (Tfh) were defined as CD45RA⁻CXCR5⁻ and CD45RA⁻CXCR5⁺ cells, respectively. Tfh could be further dissected into CXCR3⁺CCR6⁻ Tfh1, CXCR3⁻CCR6⁻ Tfh2, and CXCR3⁻CCR6⁺ Tfh17 subsets. From the memory Th cells gate, Th1, Th2, Th17, Th22, Th9, and ThG subsets were defined based on differential expression of CXCR3, CCR4, CCR6, CCR10, and CRTH2 using a gating strategy adapted from Wingender et al. 2015.
B) From the Tregs gate, memory/effector Tregs and T follicular regulatory cells (Tfr) were defined as CD45RA⁻CXCR5⁻ and CD45RA⁻CXCR5⁺ cells, respectively. Memory/effector Tregs could be further dissected into CXCR3⁺CCR6⁻ Th1-like, CXCR3⁻CCR6⁻ Th2-like, CXCR3⁻CCR6⁺CCR4⁺CCR10⁻ Th17-like and CXCR3⁻CCR6⁺CCR4⁺CCR10⁺ Th22-like subsets. Samples were acquired on a 3-laser, 12-color BD FACSLyric™ Flow Cytometer.
Final Thoughts
A balanced immune system is a healthy immune system, and understanding the mechanisms involved in fighting infections and restoring homeostasis can help clinicians provide the best care for their patients. But this is something we should all be considering for our health and well-being. Along with a balanced diet, exercise, hydration, sleep, and stress management, probiotics and nutritional supplements can play a role in immune health.
T cell profiling of the phenotypes and functions of T cells within an individual's immune system offers several clinical applications for improving the lives of patients with immune system dysfunction. At its core, T-cell profiling can help diagnose and monitor various immune-related diseases, such as autoimmune disorders, immunodeficiencies, allergies, and chronic infections. By assessing the composition and function of T cell subsets, clinicians can gain insights into the underlying immune dysregulation and disease progression and help adjust therapeutic strategies on a patient-by-patient basis, in a truly precision medicine approach.
In cancer treatment, T cell profiling is crucial for tailoring personalized cancer immunotherapies, such as checkpoint inhibitors and adoptive T cell therapies. Monitoring T-cell responses during treatment can also provide valuable information about therapy efficacy and potential resistance.
Evaluating T-cell responses to vaccination can help determine the effectiveness of vaccines and the development of long-lasting immunity. T-cell profiling provides information about the activation and memory status of vaccine-induced T cells, aiding in vaccine development and optimization.
The more we understand the complex roles CD4+ T-cells play in disease progression the more T-cell profiling can help us uncover the distinct T-cell subsets or pathways that are dysregulated in various disease conditions. This information can lead to the discovery of new therapeutic targets for drug development in the areas of infectious diseases, immunosenescence, immune reconstitution in transplant patients, and autoimmunity to name a few.
References
- Kaiko GE, Horvat JC, Beagley KW, Hansbro PM. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology. 2008 Mar;123(3):326-38. doi: 10.1111/j.1365-2567.2007.02719.x. Epub 2007 Nov 5. PMID: 17983439; PMCID: PMC2433332
- Lucey, D. R., Clerici, M. & Shearer, G. M. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin. Microbiol. Rev. 9(4), 532–562 (1996).
- Veldhoen, Marc; Uyttenhove, Catherine; van Snick, Jacques; Helmby, Helena; Westendorf, Astrid; Buer, Jan; Martin, Bruno; Wilhelm, Christoph; Stockinger, Brigitta (December 2008). "Transforming growth factor-beta 'reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset". Nature Immunology. 9 (12): 1341–1346. doi:10.1038/ni.1659. ISSN 1529-2916. PMID 18931678. S2CID 205361860
- Kaplan, Mark H. (March 2013). "Th9 cells: differentiation and disease". Immunological Reviews. 252 (1): 104–115. doi:10.1111/imr.12028. ISSN 0105-2896. PMC 3982928. PMID 23405898
- L. E. Harrington, R. D. Hatton, P. R. Mangan et al., “Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages,” Nature Immunology, vol. 6, no. 11, pp. 1123–1132, 2005.
- S. A. Khader, S. L. Gaffen, and J. K. Kolls, “Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa,” Mucosal Immunology, vol. 2, no. 5, pp. 403–411, 2009.
- Y. K. Lee, H. Turner, C. L. Maynard et al., “Late developmental plasticity in the T helper 17 lineage,” Immunity, vol. 30, no. 1, pp. 92–107, 2009.
- Workman CJ, Szymczak-Workman AL, Collison LW, Pillai MR, Vignali DA. The development and function of regulatory T cells. Cell Mol Life Sci. 2009 Aug;66(16):2603-22. doi: 10.1007/s00018-009-0026-2. Epub 2009 Apr 24. PMID: 19390784; PMCID: PMC2715449.
- Guery, L and Hugues, S. “Th17 Cell Plasticity and Functions in Cancer Immunity” BioMed Research Int. Vol. 2015. Article ID 314620. https://doi.org/10.1155/2015/314620
- Fu, W. et al. Deficiency in T follicular regulatory cells promotes autoimmunity. J. Exp. Med 215, 815–825 (2018).
- Wingender G, Kronenberg M. OMIP-030: Characterization of human T cell subsets via surface markers. Cytometry A. 2015;87(12):1067-9. doi: 10.1002/cyto.a.22788

