Blog
Written by Julie Bick, Ph.D.
Introduction
Aging is a natural process that almost all living organisms experience (the amazing ones that don’t are the topic for another blog). As the years pass, our bodies undergo a series of changes that can affect our overall health and well-being at many levels. One significant factor that has been gaining attention in recent years is the connection between inflammation and aging. Inflammation, typically a protective response by the immune system, becomes a double-edged sword as we age. This blog aims to delve into the complex interplay between inflammation and aging, shedding light on the mechanisms, implications, and potential interventions to promote healthy aging.
Understanding Inflammation
Inflammation is a highly complex and crucial defense mechanism that helps the body mitigate the effect of harmful stimuli such as pathogens, injuries, and toxins. When the immune system detects a threat, it releases various chemicals that attract immune cells to the site of injury or infection. These immune cells work together to eliminate the threat and then initiate the healing process. Acute inflammation is a short-lived response and is essential for maintaining homeostasis. However, chronic inflammation is characterized by high circulating levels of pro-inflammatory markers (e.g. IL-6, IL-8, IL-13, CRP, TNF-α) and has a much more profound effect on the aging process. Over time, factors like lifestyle, genetics, and environmental exposures can lead to a state of persistent low-level inflammation. This pervasive inflammation that occurs with advancing age is often referred to as "inflammaging" – a condition that is characterized by elevated levels of blood inflammatory markers and an increased risk of morbidity and death (Nathan and Ding, 2010).
The Mechanisms of Inflammaging
There is a growing body of evidence that inflammation and aging share a bidirectional relationship (Fulop et. al. 2018). On one hand, aging itself can lead to changes in immune system function, a phenomenon known as immunosenescence (Santoro et. al. 2021). This means that as we age, our immune system becomes less effective at responding to threats, which ultimately can contribute to persistent chronic low-grade inflammation. On the other hand, chronic inflammation can accelerate the aging process by damaging cells, tissues, and organs. This damage occurs through the production of free radicals and pro-inflammatory molecules that harm healthy cells and DNA.
Key players in the inflammation-aging connection are the chemical messengers "cytokines." These are signaling molecules, generated mainly by T-cells, that regulate inflammatory response pathways (Franceschi et. al. 2000). As we age, there is an imbalance in the production of pro-inflammatory and anti-inflammatory cytokines, tilting the scale toward inflammation. This chronic state of inflammation can affect multiple systems in the body, including the cardiovascular, nervous, and metabolic systems, and accelerating the aging process.
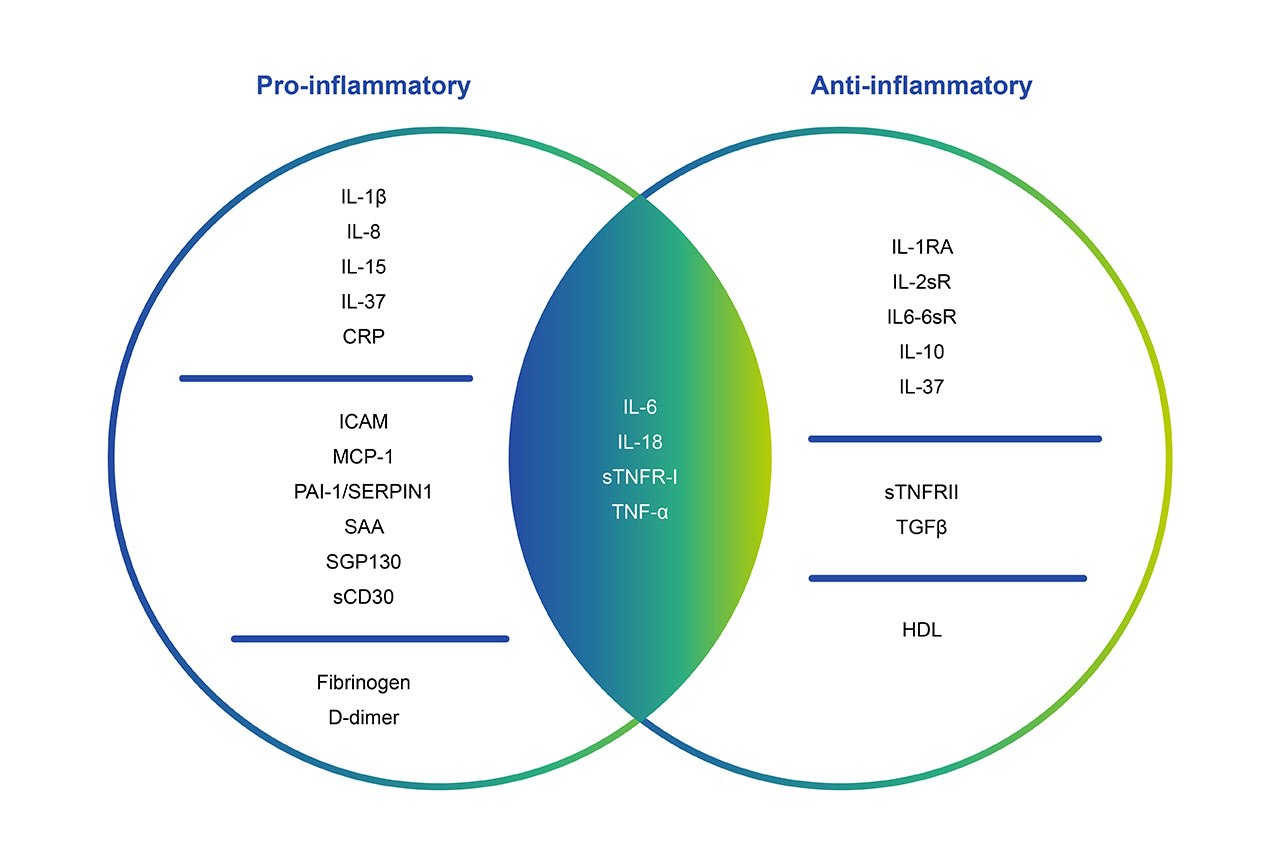
Fig. 1. Cytokines are the mediators of immune response mechanisms. They can have either pro- or anti-inflammatory functions, or act as regulators for both systems depending on age, sex or the biological context (Teissier et. al. 2022).
The Role of the Inflammasome on Aging
Cytokine profiles are only one component of inflammation-related aging, and in fact there are multiple layers of regulation involved. The term "inflammasome" is now used to refer to the complex molecular structure that plays a central role in the body's innate immune response and the regulation of inflammation. The inflammasome is essentially a molecular platform that triggers the activation of inflammatory responses when needed, using three principal components:
Pattern Recognition Receptors (PRRs)
These are proteins that recognize specific molecular patterns associated with pathogens or danger signals. PRRs are found on immune cells and serve as the first line of defense in identifying potential threats, and include Toll-like receptors, RIG-1-like receptors, NOD-like receptors, and C-type lectin receptors (Takeuchi and Akira, 2010). As we age, PRRs can become dysregulated leading to inappropriate, reduced or heightened immune responses by influencing the expression of genes that control immune system mechanisms. PRR dysregulation can result in the development of autoimmune diseases, delayed tissue healing, increased oxidative stress and imbalance in gut microbiota, all of which influences aging (Wilhelm et. al. 2017).
Adaptor Proteins
Adaptor proteins are responsible for bridging the gap between the PRRs and the effector enzyme that drives the inflammatory response mediated through immune receptors including Toll-like receptors (TLRs) and NOD-like receptors (NLRs). These adaptor proteins are essential for the proper assembly and activation of the inflammasome. While adaptor proteins themselves don't directly drive the aging process, they play a critical role in mediating and regulating cellular processes that can contribute to age-related changes and diseases (Guo et. al. 2015).
Effector Enzymes
Effector enzymes involved in processes such as DNA repair, inflammation, and oxidative stress, have a significant impact on the aging process. There is a wide variety of effector enzyme types that play essential roles in maintaining cellular and tissue function, and dysregulation of their activity can contribute to various age-related changes and diseases.
Dysfunctional or reduced activity of DNA repair enzymes can lead to increased DNA damage and mutations, accelerating the aging process and contributing to the development of age-related disorders (d’Adda di Fagagna, 2008).
Inflammatory processes are tightly regulated by effector enzymes like cyclooxygenases (COX) and phospholipase A2. Dysregulated inflammatory enzyme activity can lead to excessive production of inflammatory molecules, contributing to tissue damage, impaired function, and the progression of age-related conditions such as cardiovascular disease, neurodegenerative diseases, and metabolic disorders. Whereas antioxidant enzymes such as superoxide dismutase (SOD), catalase, and glutathione peroxidase play a crucial role in counteracting oxidative stress, which is a key contributor to the aging process (Chen et. al. 2007). Oxidative stress occurs when there is an imbalance between the production of harmful reactive oxygen species (ROS) and the body's ability to neutralize them with antioxidants. Efficient antioxidant enzyme activity helps protect cells from oxidative damage, while reduced activity can lead to cellular dysfunction, DNA damage, and accelerated aging.
Effector enzymes involved in autophagy, such as lysosomal enzymes, are crucial for the degradation and recycling of damaged or dysfunctional cellular components. Dysfunction in autophagy has been implicated in various age-related diseases, including neurodegenerative disorders, and can contribute to the accumulation of damaged molecules and organelles over time (Kale et. al. 2020). Similarly, effector enzymes involved in metabolic pathways play a role in energy production and nutrient utilization. Age-related changes in metabolic enzyme activity can impact energy production and cellular function. Dysregulation of these enzymes has been shown to contribute to metabolic disorders like diabetes and obesity, which are associated with aging and have widespread effects on overall health.
Lastly, one of the key effector enzymes of inflammasome function is caspase-1, although other caspases can also be involved. Caspases are enzymes that cleave other proteins, and in the context of the inflammasome, caspase-1 activates specific pro-inflammatory cytokines, namely interleukin-1β (IL-1β) and interleukin-18 (IL-18), by cleaving their precursor forms into their active forms (Mejias et. al. 2018).
Collectively, PRRs, adaptor proteins and effector enzymes work together to act as a molecular switch that is activated in response to various danger signals, including microbial components, cell damage, and stress-related molecules. When these danger signals are detected by PRRs, the inflammasome assembles, leading to the activation of caspase-1. Active caspase-1 then cleaves pro-IL-1β and pro-IL-18 into their active forms, which are potent pro-inflammatory cytokines. The release of IL-1β and IL-18 triggers a robust inflammatory response, leading to the recruitment of immune cells to the site of infection or tissue damage. This response helps to clear pathogens and promote tissue repair. However, when dysregulated, excessive or chronic inflammasome activation can lead to sustained inflammation, contributing to the development of various inflammatory diseases, and accelerating the aging process.
The Mechanisms of Inflammaging
Several mechanisms contribute to the connection between the inflammasome and aging, these include cellular senescence, and mitochondrial function.
Cellular senescence refers to the state in which cells lose their ability to divide and function properly. Senescent cells accumulate with age and secrete a variety of pro-inflammatory molecules, contributing to the inflammatory environment. Studies have shown that senescent cells can activate the inflammasome, leading to increased production of IL-1β and other inflammatory cytokines. This inflammasome-mediated inflammation further accelerates the aging process by promoting tissue damage and dysfunction.
With age, mitochondrial function tends to decline, leading to increased production of reactive oxygen species (ROS), which can damage cells and trigger inflammation. Dysfunctional mitochondria have been linked to inflammasome activation, particularly the NLRP3 inflammasome, which is one of the most well-studied inflammasomes. Activation of the NLRP3 inflammasome in response to mitochondrial dysfunction contributes to the release of IL-1β and IL-18, fueling the cycle of chronic inflammation.
As cells age, they accumulate cellular waste and damaged components that can trigger an immune response. The inflammasome can be activated in response to these cellular debris, further promoting inflammation. Inflammasome activation in response to accumulated waste contributes to the release of inflammatory cytokines and drives the cycle of inflammaging. As the immune system undergoes immunosenescence, its ability to regulate inflammation becomes compromised. This can lead to inappropriate or heightened inflammasome activation, contributing to the overall pro-inflammatory state. In turn, chronic inflammation can impair immune function, creating a feedback loop that exacerbates age-related inflammation.
Age-Related Lymphocyte Profiles
TBNK lymphocyte profiles (T cells, B cells, and natural killer cells) tend to undergo changes with age as part of the normal aging process of immunosenescence. The thymus, where T cells mature, undergoes involution (shrinking) with age, leading to reduced production of new naive T cells. It is Naive T cells, which are crucial for recognizing new antigens, and the decline in their number limits the immune system's ability to respond to new infections effectively. In contrast, memory T cells, which remember past infections, accumulate over time. This can be beneficial for fighting off previously encountered pathogens but may lead to a skewed immune response. Overall, the diversity of T cell receptors decreases with age, reducing the ability to recognize a wide range of antigens, and reducing the ability of the immune system to effectively respond to new challenges (Haynes et. al. 2009).
Reduced B-cell numbers are also associated with age, reducing the ability of the immune system to mount a strong antibody response to new antigens, leading to a diminished capacity to fight novel infections. Changes in B Cell Subsets are also observed, with an increase in regulatory B cells, and a decrease in Naïve B-cells, collectively these can impact the immune response's balance (Frasca and Blomberg, 2009).
NK cells, responsible for recognizing and eliminating virus-infected and cancerous cells, frequently exhibit reduced cytotoxic activity and diminished cytokine production with age that affects their overall function rather than their numbers.
These age-related changes in TBNK lymphocyte profiles can lead to an increased susceptibility to infections, reduced vaccine efficacy, and a higher risk of certain diseases like cancer. Moreover, inflammaging, can further influence immune cell function and exacerbate these changes.
Therapeutic Implications
Understanding the role of the inflammasome in aging-related inflammation opens up potential avenues for therapeutic interventions. Targeting the inflammasome caspase-1 pathway could help mitigate chronic inflammation and its negative effects on aging. Researchers are exploring various approaches, including the use of drugs that inhibit inflammasome activation or neutralize the pro-inflammatory cytokines including IL-1β and IL-18 that it produces. However, it's important to strike a balance, as some degree of inflammation is necessary for maintaining normal immune responses.
Interventions and Strategies
Lifestyle factors play a crucial role in modulating inflammation. A balanced diet rich in antioxidants, such as fruits, vegetables, and whole grains, can help counteract the effects of inflammation by neutralizing harmful free radicals. Regular physical activity has also been shown to have anti-inflammatory effects, helping to regulate immune responses and maintain healthy cellular function. Additionally, stress management and adequate sleep are integral components of a healthy lifestyle. Chronic stress can contribute to inflammation by triggering the release of stress hormones, while sleep deprivation can disrupt the body's natural anti-inflammatory processes. Incorporating relaxation techniques and ensuring sufficient sleep can have a positive impact on reducing inflammation and promoting overall well-being.
As the relationship between inflammation and aging continues to be a topic of intense research, one area of increasing interest is the role of the gut microbiota. The gut microbiota, a diverse community of microorganisms residing in the digestive tract, has been linked to immune system regulation and inflammation. Manipulating the gut microbiota through diet or probiotics could potentially modulate inflammation and promote healthy aging. Similarly, an anti-inflammatory diet has been shown to provide significant benefits in the management of inflammation. Emphasizing whole foods like fruits, vegetables, whole grains, lean proteins, and healthy fats while avoiding processed foods, sugary items, and excessive saturated fats has been clinically shown to reduce pro-inflammatory markers and support the management of inflammation related symptoms (Stromsnes et. al. 2021). Reducing caloric intake without malnutrition, along with intermittent fasting have also been shown to decrease inflammation and extend lifespan in animal studies. There are a few specific nutrients that have been shown to be highly potent in managing inflammation. These include omega-3 Fatty Acids that are found in fatty fish, flaxseeds, and walnuts; curcumin, a compound in turmeric has potent anti-inflammatory effects and may help modulate the immune response, and vitamin D.
Coupled with nutrition, exercise and physical activity can lower inflammation by promoting proper immune function and metabolic health, as well as manage stress levels and thereby mitigate inflammaging. Prioritizing good sleep hygiene is crucial, as poor sleep can lead to increased stress and inflammation; and to this end eliminating smoking and alcohol are recommended for optimal health.
Anti-Inflammatory medications including Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) can also provide benefits by reducing inflammation, but long-term use may have adverse effects. Certain biologic drugs are being researched to specifically target inflammation-related pathways. However, these are often used for specific conditions and might have side effects.
Conclusion
Chronic inflammation has been associated with a higher risk of several age-related diseases such as cardiovascular disease, diabetes, neurodegenerative disorders, and even certain types of cancer. For instance, inflammation can contribute to the development of atherosclerosis and an increased risk of heart attacks and strokes. Moreover, chronic inflammation can accelerate the decline of cognitive function. Neuroinflammation, is now known to play a role in the development and progression of conditions like Alzheimer's disease by exacerbating the accumulation of abnormal protein deposits, contributing to the death of brain cells and cognitive impairment.
Inflammation and aging share a complex and intertwined relationship that significantly impacts our health and quality of life as we grow older. Chronic inflammation, driven by a variety of factors, can accelerate the aging process and increase the risk of age-related diseases. However, through adopting a healthy lifestyle, managing stress, and staying socially active, we can potentially mitigate the effects of inflammation and promote graceful aging. As research in this field continues to advance, we may uncover even more targeted interventions that allow us to age in a way that is not only longer but also healthier and more fulfilling.
References
- Nathan C., Ding A. Nonresolving inflammation. Cell. 2010;140(6):871–882. doi: 10.1016/j.cell.2010.02.029.
- Franceschi C., Valensin S., Bonafè M., et al. The network and the remodeling theories of aging: Historical background and new perspectives. Experimental Gerontology. 2000;35(6-7):879–896. doi: 10.1016/S0531-5565(00)00172-8
- Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, Witkowski JM and Franceschi C (2018) Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 8:1960. doi: 10.3389/fimmu.2017.01960
- Santoro, A., Bientinesi, E., Monti, D. Immunosenescence and inflammaging in the aging process: age-related diseases or longevity? Ageing Research Reviews. Volume 71, 2021, 101422, ISSN 1568-1637, https://doi.org/10.1016/j.arr.2021.101422.
- Takeuchi, O., Akira, S. Pattern Recognition Receptors and Inflammation. Cell, Vol. 140, Issue 6, 2010, Pp. 805-820, ISSN 0092-8674, https://doi.org/10.1016/j.cell.2010.01.022.
- Wilhelm I, Nyúl-Tóth Á, Kozma M, Farkas AE, Krizbai IA. Role of pattern recognition receptors of the neurovascular unit in inflamm-aging. Am J Physiol Heart Circ Physiol. 2017 Nov 1;313(5):H1000-H1012. doi: 10.1152/ajpheart.00106.2017. Epub 2017 Aug 11. PMID: 28801521
- Teissier T, Boulanger E, Cox LS. Interconnections between Inflammageing and Immunosenescence during Ageing. Cells. 2022 Jan 21;11(3):359. doi: 10.3390/cells11030359. PMID: 35159168; PMCID: PMC8834134
- Guo H., Callaway J.B., Ting J.P.-Y. Inflammasomes: Mechanism of Action, Role in Disease, and Therapeutics. Nat. Med. 2015;21:677–687. doi: 10.1038/nm.3893
- d’Adda di Fagagna F. Living on a Break: Cellular Senescence as a DNA-Damage Response. Nat. Rev. Cancer. 2008;8:512–522. doi: 10.1038/nrc2440.
- Chen J.-H., Ozanne S.E., Hales C.N. Methods of Cellular Senescence Induction Using Oxidative Stress. Methods Mol. Biol. 2007;371:179–189. doi: 10.1007/978-1-59745-361-5_14.
- Kale A., Sharma A., Stolzing A., Desprez P.-Y., Campisi J. Role of Immune Cells in the Removal of Deleterious Senescent Cells. Immun. Ageing. 2020;17:16. doi: 10.1186/s12979-020-00187-9.
- Mejias NH, Martinez CC, Stephens ME, de Rivero Vaccari JP. Contribution of the inflammasome to inflammaging. J Inflamm (Lond). 2018 Nov 16;15:23. doi: 10.1186/s12950-018-0198-3. PMID: 30473634; PMCID: PMC6240324.
- Frasca D, Blomberg BB. Effects of aging on B cell function. Curr Opin Immunol. 2009;21(4):425–30
- Haynes L, Maue AC. Effects of aging on T cell function. Curr Opin Immunol. 2009;21(4):414–7.
- Stromsnes K, Correas AG, Lehmann J, Gambini J, Olaso-Gonzalez G. Anti-Inflammatory Properties of Diet: Role in Healthy Aging. Biomedicines. 2021 Jul 30;9(8):922. doi: 10.3390/biomedicines9080922. PMID: 34440125; PMCID: PMC8389628.

