Blog

Written by Julie Bick, Ph.D.
Introduction
Psychiatric disorders affect millions of people worldwide, often causing significant distress and impacting their daily lives. The current mental health crisis, with one in five adults suffering with some form of mental condition requiring medication, has steadily developed over the last 20 years (Herrman et. al. 2019). Covid exacerbated the challenges, but even over a year after the pandemic, rates of mental health conditions have not returned to pre-pandemic levels.
While the development of psychiatric drugs has been a crucial step in managing these conditions, the one-size-fits-all approach to medication has proven to be inadequate for many patients. This year, mental health medications account for around 40% of pharmacy spending for payers, and this continues to increase every year. However, there's a breakthrough on the horizon: pharmacogenomic testing is revolutionizing psychiatric medicine by tailoring treatments to individual genetic profiles. In this blog, we'll explore how pharmacogenomic testing is transforming the field of psychiatric medicine.
At its core, pharmacogenomics is the study of how an individual's genetic makeup influences their response to drugs. It considers the genetic variations of key genes that affect how a person's body metabolizes and responds to medications, enabling more effective selection of drugs and drug doses for patients, and reducing the need for polypharmacy or a prolonged cycle of different drugs before relief of symptoms. This field holds immense promise in improving the efficacy and safety of psychiatric medications and helping to alleviate the burden of mental illness on public health and healthcare resources (Jukic et. al. 2022).
Challenges of Traditional Psychiatric Medication
Traditionally, psychiatric medications have been prescribed based on a largely trial-and-error approach that can be very time-consuming and frustrating for patients who often receive little or no symptom relief. A doctor would prescribe a drug and monitor its effects, adjusting the dosage or trying a different medication if the patient did not respond positively. This process can be discouraging for patients, and it may lead to prolonged suffering, undesirable side effects, or non-compliance with the prescribed medications. Adding to this, adverse drug events involving psychiatric medications represent a major challenge to the healthcare industry, leading to an estimated 90,000 emergency room visits annually in the US, as well as increased patient morbidity and healthcare costs. This is only made worse in cases of polypharmacy, a situation that is increasingly seen in patients with chronic pain or underlying chronic conditions that then exacerbate mental health struggles (NIMH NIH 2023).
Unfortunately, more than half of patients with mental health issues have been shown not to adhere to their therapy. This is driven by several factors that include the stigma of mental illness, the affordability and availability of these drugs, and their sometimes severe side effects (Jones et, al. 2006). Sadly, this non-compliance is associated with increased severity of symptoms and decreased remission. For many patients this is intensified by substance use disorder (SUD), a condition affecting one in four adults living with mental health issues. Rates of SUD through opioid use are now slowly coming down, however, estimates of the cost of the opioid epidemic range from $1.021 to $1.5 trillion (Florence et. al. 2021). The bottom line is that the successful management of psychiatric patients requires high levels of care coordination and support, which is rarely available.
With the compounding issues of socioeconomic factors, increasing rates of homelessness and shortage of healthcare resources, there is now a drive to find new approaches and solutions that can improve not only access to psychiatric medications, but improve adherence and support to manage patient’s symptoms and hopefully reach remission.
The Power of Pharmacogenomic Testing
Every patient responds differently to a drug, and psychiatric medications are no different. Genetic profiles, along with dietary, environmental, and pathophysiological factors all influence drug responses (Perna et. al. 2020). A primary benefit of pharmacogenomic testing in psychiatric medicine is the ability to personalize treatment. By analyzing a patient's genetic profile, healthcare providers can identify which medications are most likely to be effective and which ones may cause adverse drug reactions (ADR). This individualized approach helps patients find the right treatment faster, reducing their suffering and improving their quality of life. Several key genetic variants in drug transport systems and metabolic pathways have been shown to be highly associated with psychiatric drug efficacy. Some of these genes are summarized in table 1.
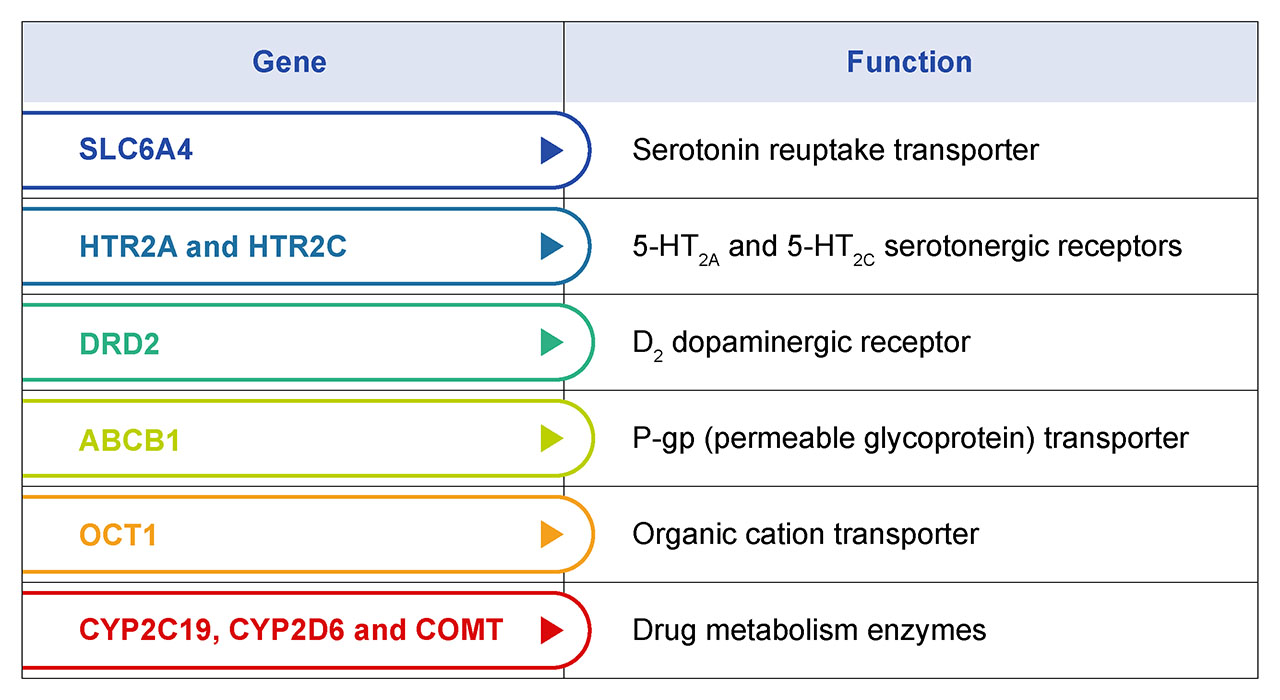
Table 1. Genes with known genetic variants that influence drug metabolism and efficacy. Collectively, they can be used to build a genetic profile of each patient that is used to describe forms for poor-, intermediate-, and ultra-rapid metabolizers. The majority of antidepressant and antipsychotic medications are processed by CYP219 and CYP2D6 encoded enzymes. Pre-emptive CYP genotyping can help select the optimal medication and dose based on the patient’s individual CYP genotypes. This works to ensure higher drug success rates through increased patient response and remission.
Pharmacogenomic testing can help predict a patient's susceptibility to medication side effects. This information allows physicians to make informed decisions when selecting medications, thereby reducing the risk of an adverse drug event and improving patient safety. It's particularly crucial in psychiatric medicine, where side effects can markedly impact a patient's mental and emotional well-being, that potentially leads to self-harm. Beyond the selection of the optimal psychiatric medications, genetic variations can also influence how a person metabolizes drugs, affecting the optimal dosage required to achieve the desired therapeutic effect. This enables healthcare providers to more rapidly determine the right dosage for each patient, reducing the risk of overmedication or undermedication, both of which can be problematic in psychiatric treatment.
The Complexity of Pharmacogenomic Testing
Much of the research on pharmacogenomics in the field of psychiatry has focused on CYP2D6, CYP3A4, CYP2C19 and CYP1A2- these encode four cytochrome P450 enzymes that are responsible for the metabolism of more than 75% of psychotropic medications (de Leon et. al. 2006). CYP2D6 displays a high variability in copy number that can have a profound effect on CYP2D6 activity in the liver and therefore on the rate of drug metabolism (Jukic et. al. 2019). Incite Health’s pharmacogenomic tests therefore include CYP2D6 copy number as a reportable for a wide range of drugs including psychotropic drugs.
There are several layers of complexity associated with pharmacogenomic profiling including the assessment of the different alleles for each of these genes that are inherited from either parent. However, databases and data processing software are now mature to the point of using comprehensive, highly multiplexed pharmacogenomic profiles to describe different drug metabolic phenotypes: poor-, intermediate-, extensive, and ultrarapid metabolizers for a range of drug classes. Examples of profiles associated with some psychiatric drugs are summarized in Table 2. Compared with extensive metabolizers with normal CYP2D6 activity, poor and intermediate metabolizers have minimal or significantly reduced activity. In contrast, ultrarapid metabolizers, who have multiple copies of CYP2D6 display high levels of activity. Depending on the drug, this phenotype may result in faster elimination of the drug reducing its efficacy, or the processing of the pro-drug form too rapidly potentially to the point of toxicity meaning that drug is not suitable for that patient. State-of-the-art pharmacogenomic reporting captures this information to provide clinically powerful recommendations for drug selection and dosing for that individual across a range of medication classes.
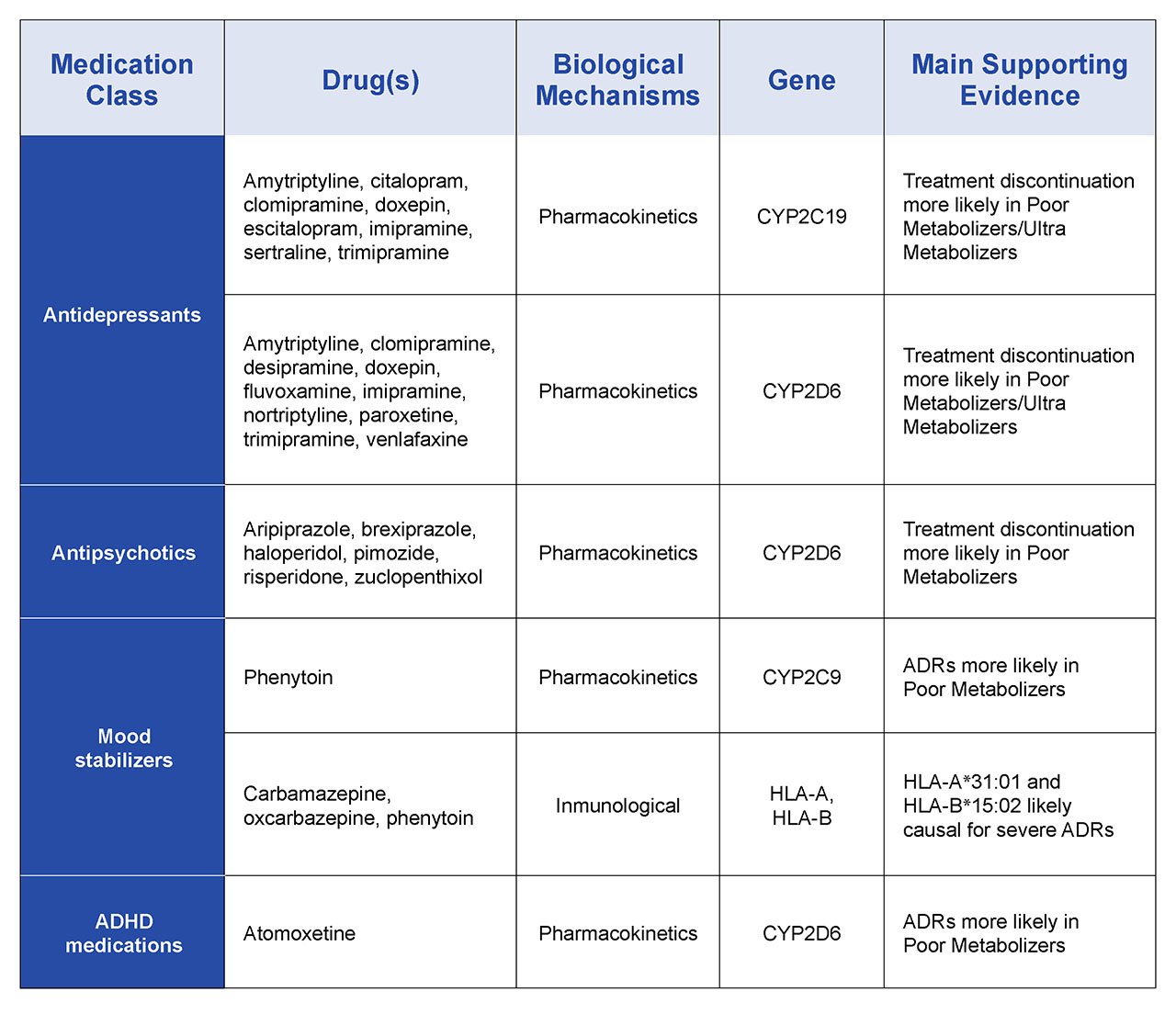
Table 2. Examples of psychiatric pharmacogenomic drug-gene associations reflected in current PharmGKB-annotated guidelines.
Challenges and Future Developments
Pharmacogenomic testing has already made a significant impact on psychiatric medicine. Several case studies and anecdotal evidence have shown remarkable improvements in patient outcomes. Patients who had previously struggled with medication resistance or debilitating side effects have found relief through pharmacogenomic testing. Moreover, ongoing research and clinical trials continue to expand our understanding of how genetic information can be leveraged to improve psychiatric care. As the field evolves, it's likely that we'll see even more effective treatments and a reduction in the stigma associated with psychiatric disorders.
However, while pharmacogenomic testing holds great promise, it is not without challenges. Some of the hurdles include the cost of testing, limited insurance coverage, and the need for greater awareness and training among healthcare providers. In addition, much of the work to date has involved limited ethnic backgrounds; this is significant since the frequency of different genetic alleles within different ethnic groups varies a great deal, and although there is a push to diversify patient groups within clinical trials, it is well known that many ethnic groups are underrepresented in clinical studies. As a result, there are calls to establish well-defined categories beyond black, white and Asian to be used as a way to improve precision in pharmacogenomic research and support the expansion of more relevant and accurate databases. Additionally, pharmacogenomic profiling alone doesn’t consider the effects of age, epigenetics, sex, Body Mass Index (BMI) of patients, all factors that may impact drug absorption and metabolism (Merrill et. al. 2019).
As this technology advances, and more clinical studies are published, it is believed that pharmacogenomic profiling will become more widely accepted among all stakeholders, and the current obstacles to pharmacogenomic testing are expected to diminish.
There are three major groups championing the use of pharmacogenomic testing for psychiatric patients- PharmGKB, a group funded by the NIH that evaluates clinical gene-drug evidence and publishes guidelines for clinicians to tap into when designing therapeutic strategies, and then the Clinical Pharmacogenomics Information Consortium (CPIC), an international organization that supports similar initiatives. Incite Health implements up to date CPIC guidelines into all of the organization’s pharmacogenomic reports. Lastly, The International Society of Psychiatric Genetics (ISPG) focuses only on pharmacogenomic recommendations for psychiatric medications. Unfortunately, there are some instances where the gene-drug recommendations do not align across these organizations- this is largely due to inconsistencies in the sources of clinical data, or even mission statements of the sources (Shugg et. al. 2022) meaning that threshold data for a given drug-gene effect was not consistent across the different organizations. It is evident that to best serve patients, there is a requirement for comprehensive review and consensus of pharmacogenomic testing in psychiatry (Bousman et. al. 2021)
All of this is now being supported by the publication of several key retrospective studies in psychiatric medicine, where patient’s pharmacogenomic profiles were used to build out optimal treatment programs- that are then compared with the treatments that the patients had actually received and the clinical outcome from that treatment (Ahmed et. al. 2021; Roberts et. al. 2021; Brown et. al. 2021). These studies consistently show a significant benefit to pharmacogenomic-driven therapeutic decision making, and have identified unwarranted and potentially harmful polypharmacy, and clearly demonstrated improved clinical outcome when the pharmacogenomic identified therapeutic strategy was aligned with the actual treatment. More studies like these are needed in order for us to really understand the value and the limitations of pharmacogenomics and will be important in establishing a portfolio of evidence-based gene-drug interaction.
Final Thoughts
In recent years there has been incredible progress in the field of pharmacogenomics, with a significant increase in studies and clinical trials, the potential of this testing to transform patient lives is evident (Malhotra et. al. 2012; Pardiñas et. al. 2021). Pharmacogenomic testing is transforming psychiatric medicine by ushering in a new era of personalized, effective, and safe treatments. By tailoring medications to individual genetic profiles, this approach is addressing the shortcomings of traditional psychiatric therapeutic practices. As awareness grows and technology advances, we can look forward to a future where psychiatric disorders are managed with greater precision and compassion, ultimately enhancing the well-being of individuals living with these conditions.
References
- Herrman H., Kieling C., McGorry P., Horton R., Sargent J., Patel V. Reducing the global burden of depression: A Lancet–World Psychiatric Association Commission. Lancet. 2019;393:e42–e43. doi: 10.1016/S0140-6736(18)32408-5.
- Jukic M., Milosavljevic F., Molden E., Ingelman-Sundberg M. Pharmacogenomics in treatment of depression and psychosis: an update. Trends Pharmacol Sci. 2022;43(12). doi: 10.1016/j.tips.2022.09.011.
- National Institute of Mental Health. Mental Illness. https://www.nimh.nih.gov/health/statistics/mental-illness. Published March 2023. Accessed April 15, 2023.
- Jones PB, Barnes TRE, Davies L, et al. Randomized Controlled Trial of the Effect on Quality of Life of Second- vs First-Generation Antipsychotic Drugs in Schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Psychiatry. 2006;63(10):1079–1087. doi:10.1001/archpsyc.63.10.1079
- Florence, C., Luo, F., & Rice, K. (2021). The economic burden of opioid use disorder and fatal opioid overdose in the United States, 2017. Drug and Alcohol Dependence 218 (2021). Accessed on line on 1/14/2021
- Perna,G., Alciati, A., Dacco, S., Grassi, M., Caldirola, D. (2020) Personalized Psychiatry and Depression: The Role of Sociodemographic and Clinical Variables. Psychiatry Investigation 2020;17(3):193-206. March 12, 2020 DOI: https://doi.org/10.30773/pi.2019.0289
- de Leon, Jose, Scott C. Armstrong, and Kelly L. Cozza. "Clinical guidelines for psychiatrists for the use of pharmacogenetic testing for CYP450 2D6 and CYP450 2C19." Psychosomatics 47.1 (2006): 75-85.
- Marin M Jukic; Robert L Smith, Tore Haslemo, Espen Molden, Magnus Ingelman-Sundberg. Effect of CYP2D6 genotype on exposure and efficacy of risperidone and aripiprazole: a retrospective, cohort study. The Lancet Psychiatry. VOLUME 6, ISSUE 5, P418-426, MAY 2019. DOI: https://doi.org/10.1016/S2215-0366(19)30088-4
- Malhotra AK, Zhang JP, Lencz T. Pharmacogenetics in psychiatry: translating research into clinical practice. Mol Psychiatry. 2012 Jul;17(8):760-9. doi: 10.1038/mp.2011.146. Epub 2011 Nov 15. PMID: 22083729; PMCID: PMC3928114.
- Antonio F. Pardiñas, Michael J. Owen, James T.R. Walters. Pharmacogenomics: A road ahead for precision medicine in psychiatry. Neuron Volume 109, Issue 24, 2021, pp3914-3929, ISSN 0896-6273, https://doi.org/10.1016/j.neuron.2021.09.011.
- Merrill RM, Fowers R. To what extent does sex, age and BMI impact medical and pharmacy costs? A retrospective cohort study involving employees in a large school district in the USA. BMJ Open. 2019 May 28;9(5):e024078. doi: 10.1136/bmjopen-2018-024078. PMID: 31142517; PMCID: PMC6549653.
- Shugg T, Pasternak AL, Luzum JA. Comparison of clinical pharmacogenetic recommendations across therapeutic areas. Pharmacogenet Genomics. 2022 Feb 1;32(2):51-59. doi: 10.1097/FPC.0000000000000452. PMID: 34412102; PMCID: PMC8702450.
- Bousman CA, Bengesser SA, Aitchison KJ, Amare AT, Aschauer H, Baune BT, Asl BB, Bishop JR, Burmeister M, Chaumette B, Chen LS, Cordner ZA, Deckert J, Degenhardt F, DeLisi LE, Folkersen L, Kennedy JL, Klein TE, McClay JL, McMahon FJ, Musil R, Saccone NL, Sangkuhl K, Stowe RM, Tan EC, Tiwari AK, Zai CC, Zai G,
- Ahmed S, Tahir R, Akhtar U, Faiz M. Pharmacogenomics Guided Prescription Changes Improved Medication Effectiveness in Patients With Mental Health-Related Disability: A Retrospective Cohort Analyses. Front Genet. 2021 Aug 3;12:644694. doi: 10.3389/fgene.2021.644694. PMID: 34413872; PMCID: PMC8370841.
- Roberts TA, Wagner JA, Sandritter T, Black BT, Gaedigk A, Stancil SL. Retrospective Review of Pharmacogenetic Testing at an Academic Children's Hospital. Clin Transl Sci. 2021 Jan;14(1):412-421. doi: 10.1111/cts.12895. Epub 2020 Oct 15. PMID: 33048453; PMCID: PMC7877836.
- Brown, L.; Li, J.; Katel, N.; Yu, K.; Fatourou, E.; Himmler, B.; Halaris, A. Pharmacogenetic Testing in an Academic Psychiatric Clinic: A Retrospective Chart Review. J. Pers. Med. 2021, 11, 896. https://doi.org/10.3390/jpm11090896
- Zhang J, Gaedigk A, Müller DJ. Review and Consensus on Pharmacogenomic Testing in Psychiatry. Pharmacopsychiatry. 2021 Jan;54(1):5-17. doi: 10.1055/a-1288-1061. Epub 2020 Nov 4. PMID: 33147643.

