Blog

Written by Julie Bick, Ph.D.
Pharmacogenomics, the study of how an individual's genetic makeup influences drug responses, has ushered in a new era of personalized medicine. One of the areas most impacted by pharmacogenomics is within the field of clinical trials. Traditionally, clinical trials followed a one-size-fits-all approach, treating all patients as a homogeneous group. However, with the integration of pharmacogenomics, clinical trials have become more targeted and tailored, enabling researchers to identify subgroups of patients who are more likely to respond positively to specific treatments, and eliminating those at risk of adverse drug reaction.
In this blog, we will explore the many ways in which pharmacogenomics is reshaping the clinical trial landscape, from study protocol design to patient selection and ultimately to safer treatment optimization.
The Many Challenges of Clinical Trial Design
Anyone involved in clinical trials knows how complex and challenging they can be. From the study design to patient enrollment, and the collation of data, the overall logistics can be very demanding. As an effort to address this, the implementation of pharmacogenomics is revolutionizing the way clinical trials are designed and implemented, by stratifying patients based on genetic profiles. Using genomic information, researchers can identify specific genetic markers/biomarkers that may affect drug responses and safety profiles allowing for the stratification of patients into more homogenous subgroups, that ultimately helps to maximize the statistical power of the trial and improving the chances of detecting meaningful treatment effects.
Patient Enrollment
Patient enrollment is one of the most significant and complex challenges of most clinical trials. There are a range of reasons for this from simple awareness and education around trial availability to the geographical and cultural barriers that some patients face dealing with clinical research organizations. But for many patients, there is a significant burden in navigating the eligibility criteria for clinical trials. By pre-screening potential participants for relevant genetic markers, researchers can identify individuals who are more likely to benefit from the investigational drug. This approach streamlines the recruitment process, reduces costs and shortens the time required to enroll the minimal required number of eligible patients. This strategy has benefited trials targeting rare diseases the most; typically, this type of trial has a very limited patient populations for enrollment- identifying those most likely to benefit from the new therapeutic benefits all stakeholders.
Dosing
In early-phase clinical trials, where ‘safety’ and ‘tolerability’ are primary endpoints, pharmacogenomics plays a crucial role in identifying potential responders, non-responders and those who might be most at risk of adverse drug reactions who might require more carefully monitoring. By analyzing genomic data from patients experiencing positive treatment outcomes, researchers can gain insights into potential genetic biomarkers that predict drug efficacy, guiding dose-finding studies and subsequent phases of clinical development (Hockings, et. al. 2020).
Pharmacogenomics also provides valuable information on how an individual's genetic makeup affects drug metabolism and response. This knowledge allows researchers to optimize drug dosages and treatment regimens based on a patient's genetic and therefore metabolic profile. By tailoring dosages to match an individual's specific metabolic capacity, clinical trials can avoid under- or over-treatment, leading to more effective and safer drug administration.
In parallel with profiling how a patient will metabolize drug molecules, we can use this information to determine if either slow or enhanced metabolic rate may create potential toxicity or lead to an adverse reaction to the administered drug or metabolized drug product. Adverse drug reactions (ADRs) are a significant concern in clinical trials, and beyond drug approval. ADRs account for a staggering number of ER visits, hospitalizations and costly healthcare resources reportedly as much as $30B annually (Sultana et. al. 2013). But beyond the cost, ADRs cost lives, and therefore pharmacovigilance programs have to this point been considered critical in mitigating the burden of ADRs on patient safety and on the healthcare system. Pharmacogenomics enables researchers to identify genetic markers associated with increased susceptibility to ADRs, therefore by screening participants for these markers before the trial begins, researchers can exclude individuals at higher risk of adverse reactions or design appropriate safety measures, ensuring patient safety and reducing potential trial disruptions.
Adaptive Clinical Trial Design.
Pharmacogenomics has influenced the adoption of so called ‘adaptive clinical trial designs’. In adaptive trials, interim data analyses are used to modify the study parameters based on emerging results in accordance with pre-specified rules (Pallmann et. al. 2018). By incorporating pharmacogenomic data into these analyses, researchers can adapt the trial to focus on patient subgroups showing the most promising responses to the drug, thereby increasing the chances of success, and reducing the need for resource-intensive large-scale trials. Unfortunately, and possibly due to the substantial statistical requirements required to run this type of study, the pace of adoption has been slower than expected. However, the CDC has recently provided more context around the design, terminology and ethics involved in this type of strategy which is helping to provide practical solutions towards a more mainstream implementation.
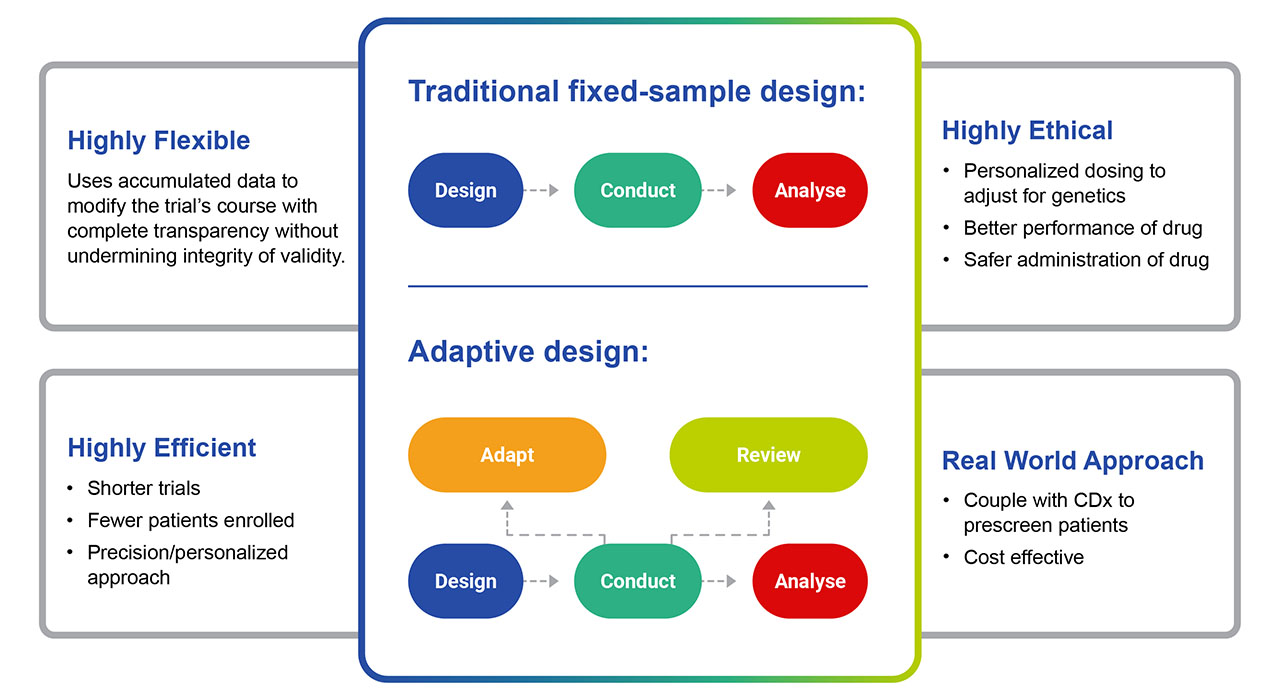
Fig. 1. The benefits of Adaptive Clinical Trial Design versus Traditional. Enhances performance of the trial, increases the timeframe to clinical trial conclusion, lowers costs and provides a safer path to approval.
A Path to Personalized Medicine with Companion Diagnostics.
Companion diagnostics (CDx) tests are designed to identify patients that are most likely to respond positively to a specific drug. CDx tests have become an integral part of pharmacogenomics-informed clinical trials as well as the post-approval phases of drug development. These tests are used to identify patients with specific genetic profiles that are associated with beneficial treatment outcomes. The integration of companion diagnostics into clinical trial design ensures that the study population is enriched with individuals who are likely to respond to the drug, increasing the likelihood of a successful trial outcome. This not only improves the safety of clinical trials, but can lead to smaller and more efficient trials, with reduced costs and timeline to approval.
Improved Pharmacokinetic and Pharmacodynamic Studies.
Collectively, pharmacokinetic and pharmacodynamic studies are used to describe how a drug functions within the body. At a high level, pharmacokinetic studies examine how a drug is absorbed, distributed, metabolized, and eliminated from the body; whereas pharmacodynamic studies analyze how drugs interact with their target molecules and establish a therapeutic effect via a so called ‘mechanism of action’. It is a highly complex process, and the FDA requires this level of examination before even the most efficacious drug molecules are approved. Understanding how a patient's genetics influence drug metabolism and drug-target interactions allows researchers to gain more precise insights into drug behavior within the body. This knowledge aids in optimizing targeted drug exposure, by predicting the absorption and metabolic rates that influence the distribution of the drug in the targeted tissues as well as to other tissues that might result in side effects. In addition, these studies profile the length of the drug’s time in circulation and the rate at which the body eliminates the drug and drug products. All of this information is collated to more comprehensively evaluate the drug efficacy, safety profiles that are used to guide dosing schedules that ultimately enhance patient outcomes.
Drug Repurposing and Combination Therapies
Over the past decade, pharmacogenomics has facilitated the identification of genetic markers that can be targeted by existing drugs approved for other indications. This knowledge has led to drug repurposing efforts, where existing medications are investigated for their potential efficacy in treating different diseases based on their genetic targets. Drug repurposing has many advantages from significant cost savings to more rapid FDA approval. In recent years, around 30% of new drug approvals are for repurposed drugs, accounting for about 25% of the industries revenue (Cha et. al. 2018). No area of medicine does this offer the most potential than in the development of drugs for the 6000 or so rare, neglected, and orphan diseases (Pushpakom et al. 2019). The coupling of pharmacogenomics with AI is now opening the possibilities of developing combination therapies to address these unmet medical needs in a way that is economically feasible and safer. In this regard, pharmacogenomics has paved the way for combination therapies, where multiple drugs targeting different genetic pathways are used simultaneously to enhance treatment efficacy (Schaefer et. al. 2020).
Pediatric Clinical Trials
Despite its high degree of clinical relevance, pharmacogenomic testing in pediatric medicine has been limited. The Netherlands and Canada lead the global implementation of pediatric testing, but there are well-recognized barriers to its adoption, particularly for off-label prescribing. Despite limited data sets, pharmacogenomics has facilitated the inclusion of children into clinical trials, but largely based on the extrapolation of larger adult-derived datasets. This knowledge gap is being addressed through international-, interdisciplinary collaborations. It is evident that children often require specialized treatment approaches, and using the data from clinical research networks, researchers are now able to identify genetic markers specific to pediatric populations, optimizing treatment regimens and ensuring children receive tailored therapies that are both effective and safe (Barker et. al. 2022).
Final Thoughts
There is no doubt that pharmacogenomics has shifted the focus of clinical trials towards patient-centric approaches. By customizing treatment based on individual genetic profiles, researchers can improve patient outcomes and experiences. In addition, patients are more likely to be willing to participate in clinical trials when they know they are receiving treatments tailored to their unique genetic makeup, fostering greater patient engagement and compliance. These modest adjustments, are revolutionizing clinical trials, transforming the way drugs are developed and tested. From enhanced study design and patient stratification to optimized drug dosages and improved safety, pharmacogenomics is maximizing the efficiency and success of clinical trials, and leading to more effective and personalized treatments.
As pharmacogenomics continues to advance, its impact on clinical trials is only expected to grow, driving the shift towards patient-centric precision medicine and unlocking the potential for improved treatment outcomes across a wide range of diseases.
References
- Jennifer K. Hockings, PharmD, PhD, Amy L. Pasternak, PharmD, Angelika L. Erwin, MD, PhD, Neil T. Mason, MBA, Charis Eng, MD, PhD and J. Kevin Hicks, PharmD, PhD. Pharmacogenomics: An evolving clinical tool for precision medicine. Cleveland Clinic Journal of Medicine February 2020, 87 (2) 91-99; DOI: https://doi.org/10.3949/ccjm.87a.19073
- Sultana J, Cutroneo P, Trifirò G. Clinical and economic burden of adverse drug reactions. J Pharmacol Pharmacother. 2013 Dec;4(Suppl 1):S73-7. doi: 10.4103/0976-500X.120957. PMID: 24347988; PMCID: PMC3853675.
- Pallmann, P., Bedding, A.W., Choodari-Oskooei, B. et al. Adaptive designs in clinical trials: why use them, and how to run and report them. BMC Med 16, 29 (2018). https://doi.org/10.1186/s12916-018-1017-7
- Cha Y, Erez T, Reynolds IJ, Kumar D, Ross J, Koytiger G, et al. Drug repurposing from the perspective of pharmaceutical companies. British Journal of Pharmacology. 2018;175(2):168-180
- Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, et al. Drug repurposing: Progress, challenges and recommendations. Nature Reviews. Drug Discovery. 2019;18(1):41-58
- Schaefer, J., Lehne, M., Schepers, J. et al. The use of machine learning in rare diseases: a scoping review. Orphanet J Rare Dis 15, 145 (2020). https://doi.org/10.1186/s13023-020-01424-6
- Barker CIS, Groeneweg G, Maitland-van der Zee AH, Rieder MJ, Hawcutt DB, Hubbard TJ, Swen JJ, Carleton BC. Pharmacogenomic testing in paediatrics: Clinical implementation strategies. Br J Clin Pharmacol. 2022 Oct;88(10):4297-4310. doi: 10.1111/bcp.15181. Epub 2022 Feb 7. PMID: 34907575; PMCID: PMC9544158.

