Blog

Written by Julie Bick, Ph.D.
Flow cytometry is a powerful technique used to analyze cells based on their surface and intracellular markers that are detected using specific antibodies that bind to these biomarkers. For clinical applications the performance and reproducibility of the analysis are critical. There are therefore key differences in performing flow cytometry for clinical diagnostic applications, compared with research and development programs (Pedreira, C. E. et. al. 2013). In this blog, we explore the differences in reagents, instrumentation, panel design, and validation, coupled with the regulatory requirements that distinguish clinical flow cytometry.
Clinical Flow Cytometry Instrumentation
IVD (In Vitro Diagnostics) flow cytometers and research-grade flow cytometers differ primarily in their intended use and design specifications. IVD flow cytometers are specifically designed and validated for clinical use and are used in diagnostic laboratories to analyze patient samples for various medical conditions. They have stringent requirements for accuracy, precision, and reliability, as well as regulatory compliance, to ensure that the results are consistent and reproducible. They also often have built-in quality control measures and software to help ensure the accuracy and reliability of results by including automated gating and reporting features (Ferbas J, Schroeder MJ, 2010). In contrast, research-grade flow cytometers are designed for research use and often have a wider range of applications and flexibility. They are typically used in research laboratories to analyze cell populations for basic research, drug discovery, and development applications. They often have a higher sensitivity and resolution than IVD flow cytometers, which may allow for a more detailed analysis of cellular populations, but they may not meet the stringent regulatory requirements needed for diagnostic testing and are frequently less stable in performance from day to day.Clinical Grade Reagent Selection and Optimization
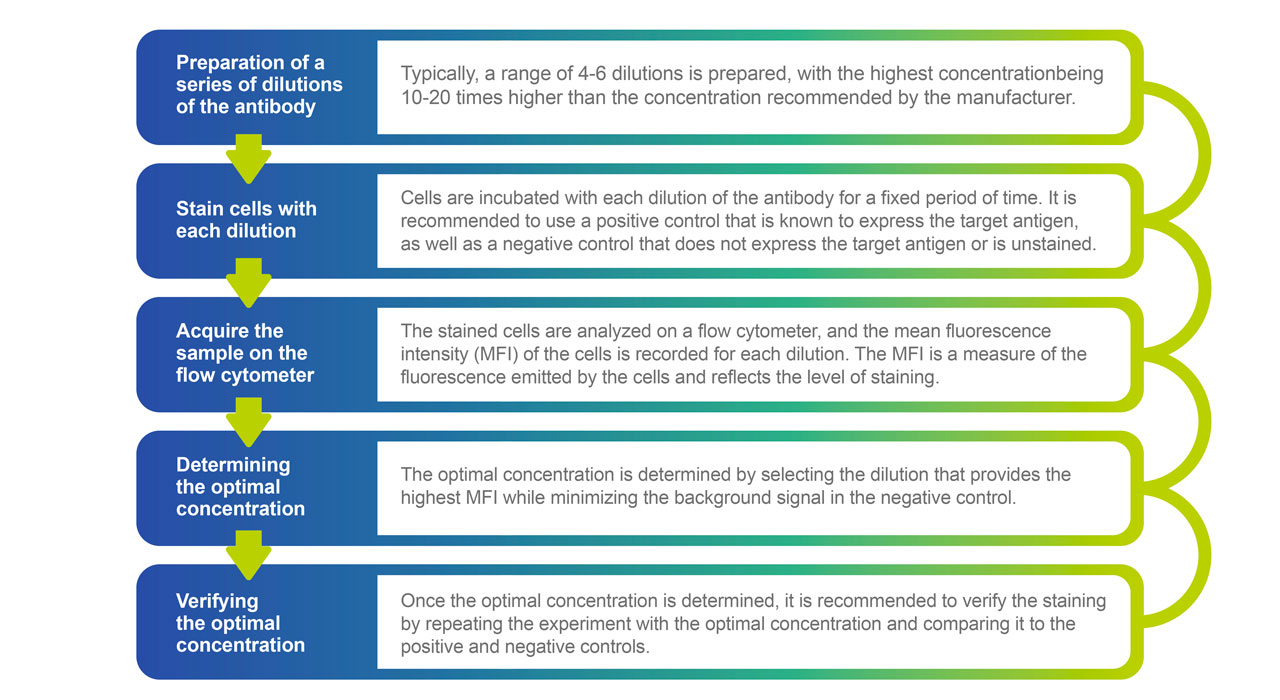
IVD (In Vitro Diagnostic) grade antibodies for flow cytometry are specifically designed and manufactured for clinical use and are subject to stringent quality control and regulatory compliance. These antibodies must meet specific performance standards and be manufactured according to strict guidelines to ensure consistency and reliability of results. IVD-grade antibodies are validated for use in diagnostic assays and must meet specific criteria for sensitivity, specificity, and reproducibility. (Wu DY, et. al. 2010). In contrast, research-grade antibodies are not subject to the same regulatory requirements and may not meet the same level of quality control as IVD-grade antibodies. Research-grade antibodies are intended for use in research settings, where reproducibility and accuracy are important but not subject to the same level of regulatory oversight as clinical diagnostics. They typically have a wider range of applications and do not have the same level of validation and quality control as IVD-grade antibodies. Between these two grades of antibodies, there is the designation for Analyte Specific Reagent (ASR), which is based on 21CFR864.4020 FDA Guidelines. ASR reagents have some form of Quality Control and are intended for use in diagnostic applications but must be validated within the laboratory being used and for the specific flow cytometry analysis. Antibody titration is a critical step in optimizing the use of antibodies in all flow cytometry panels to ensure accurate and reproducible results. The goal of antibody titration is to determine the optimal concentration of the antibody that will provide the best staining and signal-to-noise ratio for the cells of interest. All reagents and antibodies used for clinical flow cytometry must be optimized and titrated. There are several steps involved in the titration of antibodies for flow cytometry analysis:
Clinical Panel Design
Designing a clinical flow cytometry panel involves a series of steps to select the appropriate antibodies, fluorochromes, and controls to identify and quantify specific cell populations or biomarkers in patient samples. As a general rule, the lower the number of colors, the higher the performance and reliability of the panel, and most clinical flow cytometry panels are 3-10 colors (Wood B. 2006). In addition, important considerations include the selection of fluorochrome-antibody combinations to minimize the need for compensation and optimize biomarker detection from each laser. There are some general considerations when designing a clinical flow cytometry panel:What is the clinical objective for the panel?
The first step in designing a clinical flow cytometry panel is to clearly define the clinical question or hypothesis that the panel is intended to address. This involves identifying the patient population, the specific disease or condition, and the relevant biomarkers or cell populations of interest.Are there optimal antibody clones?
Once the clinical question is defined, the next step is to select the appropriate antibody clones for the panel. Antibodies are selected based on their specificity for the target biomarkers and their ability to work in the context of flow cytometry. It is important to consider factors such as cross-reactivity, background staining, and optimal concentration when selecting antibodies. Typically, IgG1 and IgG2a isotypes are preferred due to their superior binding characteristics.Are there optimal fluorochromes for each of the antibodies?
Fluorochromes are selected based on their excitation and emission spectra and their compatibility with the chosen antibodies. It is important to choose fluorochromes that are bright and stable, and that minimize spectral overlap to allow for accurate identification of cell populations. Ideally, the brightest fluorochromes should be used to detect the least abundant markers. Caution should be taken when using tandem dyes due to stability issues, and it is also important to take compatibility with staining buffers into account to minimize non-specific binding.Design the staining protocol
Once the antibodies and fluorochromes are selected, the next step is to design the staining protocol. This involves optimizing the concentration of the antibodies and fluorochromes, determining the appropriate incubation times and temperatures, and selecting appropriate washing and fixation steps. These are then standardized to ensure the reproducibility of the assay across days, clinical technicians, and instruments.Choose the controls
Controls are an essential component of a flow cytometry panel and are used to assess the performance of the assay and to determine reference ranges for the biomarkers of interest. Positive and negative controls should be selected to validate the panel and to ensure that the assay is working correctly, this may be in conjunction with calibration beads for MFI reporting, and internal controls for activation markers for example (Tung JW, et. al. 2007; Hulspas R, 2009).Optimize the gating strategy
Gating is the process of selecting the cell population of interest from the total cell population based on their staining characteristics. The gating strategy should be optimized to ensure that the identified population is representative of the target cell population and to minimize non-specific staining and background noise.Validate the panel
Validate the panel Once the panel is designed, it should be validated using appropriate quality control measures. Validation involves testing the panel on known positive and negative controls, conducting precision and accuracy testing, and establishing reference ranges of antigen-specific signals for the biomarkers of interest (Hulspas R, et. al. 2009).Validation of a Clinical Flow Cytometry Panel
Validation of a clinical flow cytometry panel is a critical step in ensuring the accuracy and reliability of flow cytometry results for diagnostic purposes. The validation process involves a series of tests and quality control measures to assess the performance of the flow cytometry panel and ensure that it meets the required specifications and standards. The first step involves clearly defining the clinical question that the panel is intended to address along with the critical cell populations and endpoints. Keeping the panel as streamlined as possible can only help in the performance of the panel in a clinical setting. Designing the panel involves selecting the appropriate antibodies and fluorochromes, determining the optimal staining conditions, and designing gating strategies for data analysis. The panel performance is typically then verified by assessing the panel on known positive and negative controls to confirm that it can identify the target biomarkers and discriminate between positive and negative populations. Endpoint Validation is then performed to assess precision and accuracy (Cummings J, et. al. 2010).Conduct precision testing
Precision testing is designed to evaluate the reproducibility of the panel performance. This involves running multiple replicates of the same sample and comparing the results to assess the consistency of the measurements.Conduct inter-laboratory testing
Inter-laboratory testing is designed to evaluate the consistency of the panel performance across different laboratories. This involves testing the panel on the same samples in different laboratories and comparing the results.Establish reference ranges
Once the panel performance has been validated, reference ranges can be established for the biomarkers of interest. This involves analyzing samples from a healthy population to determine the normal range of expression levels for each biomarker.Proficiency Testing Materials
Proficiency testing materials are used in clinical flow cytometry to assess the performance of a laboratory or instrument operator. Proficiency testing is an important component of quality control and is required by regulatory agencies such as the Clinical Laboratory Improvement Amendments (CLIA) and the College of American Pathologists (CAP) for accreditation. Proficiency testing materials are usually provided by a third-party organization that specializes in producing quality control materials. These materials are designed to simulate real-world samples and may contain a mixture of cell types with varying expression levels of specific antigens. A clinical flow cytometry laboratory will receive a shipment of samples on a regular schedule and process these using their standard operating procedures for flow cytometry analysis. The results are then submitted to the third-party organization for evaluation which includes comparing the laboratory's results to a predefined target value or assessing the laboratory's overall performance based on a set of criteria. Based on the evaluation, the third-party organization may provide feedback to the laboratory on areas where improvements can be made or provide suggestions for further training or education.Proficiency testing programs are a valuable tool for clinical flow cytometry laboratories to ensure the accuracy and reliability of their results and to maintain compliance with regulatory requirements.Final Thoughts
Designing and executing a clinical flow cytometry panel is a complex process that requires careful consideration of the clinical question, antibody and fluorochrome selection, staining protocol, control selection, gating strategy, and validation steps. A well-designed panel is essential for accurate and reliable identification and quantification of specific cell populations or biomarkers in patient samples. Once the assay is validated for clinical applications, it is important that the performance of the panel can be monitored over time using standardized control test materials, and lot-to-lot comparisons of all critical reagents performed as needed (Bottcher, S. et. al. 2017). Flow Cytometry IVD instrumentation is designed to provide the stability and automation required for high-throughput analysis that maintains the standards for clinical testing for diagnostic applications. Incite Health performs all clinical flow cytometry assays following the CLIA and CAP regulations for good clinical practices and principles that ensure the highest quality of data from your patient’s samples. In addition, we follow the guidelines recommended by the Clinical Laboratory Standards Institute (CLSI) and are contributing partners of the NIST Flow Cytometry Consortium we are working to achieve harmonization and standardization of clinical flow cytometry to help minimize inter-laboratory, inter-instrument, and inter-operator variability in flow cytometry, cell-based analyses. With our expertise in clinical flow cytometry and established procedures for immune system monitoring, we are the partner of choice for developing your clinical flow cytometry assays and companion diagnostics.References:
- Pedreira, C. E. et al. Overview of clinical flow cytometry data analysis: recent advances and future challenges. Trends Biotechnol. 31, 415–425. https://doi.org/10.1016/j.tibtech.2013.04.008 (2013).
- Ferbas J, Schroeder MJ. Instrument validation for regulated studies. In: Flow Cytometry in Drug Discovery and Development. Litwin V, Marder P (Eds). John Wiley & Sons Inc., NJ, USA, 267–277 (2010).
- Wood B. 9-color and 10-color flow cytometry in the clinical laboratory. Arch. Pathol. Lab. Med. 130(5), 680–690 (2006)
- Wu DY, Patti-Diaz L, Hill CG. Development and validation of flow cytometry methods for pharmacodynamic clinical biomarkers. Bioanalysis 2(9), 1617–1626 (2010)
- Tung JW, Heydari K, Tirouvanziam R, Sahaf B, Parks DR, Herzenberg LA. Modern flow cytometry: a practical approach. Clin. Lab. Med. 27(3), 453–468; v (2007).
- Hulspas R, O'Gorman MR, Wood BL, Gratama JW, Sutherland DR. Considerations for the control of background fluorescence in clinical flow cytometry. Cytometry B Clin. Cytom. 76(6), 355–364 (2009)
- Cummings J, Raynaud F, Jones L, Sugar R, Dive C. Fit-for-purpose biomarker method validation for application in clinical trials of anticancer drugs. Br. J. Cancer 103(9), 1313–1317 (2010)
- Hill C, Wu D, Ferbas J, Litwin V, Reddy M. Regulatory compliance and method validation. In: Flow Cytometry in Drug Discovery and Development. Litwin V, Marder P (Eds). John Wiley & Sons, Inc., NJ, USA, 243–266 (2010)
- Bottcher, S. et al. Lot-to-lot stability of antibody reagents for flow cytometry. J. Immunol. Methods. https://doi.org/10.1016/j.jim.2017.03.018 (2017)

